Effects of exogenous melatonin on the morphology and antioxidant enzyme activities of cotton seedlings under salt stress
-
摘要: 褪黑素是一种有效的抗氧化剂, 能够促进逆境胁迫下植物的生长发育, 减缓逆境胁迫产生的伤害。棉花是我国重要的经济作物, 盐胁迫严重影响其生长发育。为探究褪黑素对盐胁迫下棉花生长发育的调控效应, 本研究以‘国欣棉9号’为材料, 设置不同浓度褪黑素浸种处理, 对盐胁迫下(150 mmol∙L−1 NaCl)棉苗根系形态(总根长、总表面积、总体积、侧根数目、主根长、主根表面积、主根平均直径)进行了测定, 筛选出适宜的褪黑素浓度(10 μmol∙L−1); 并分析正常生长条件(CK)、10 μmol∙L−1褪黑素浸种(MT)、150 mmol∙L−1 NaCl处理(S)、10 μmol∙L−1褪黑素浸种和150 mmol∙L−1 NaCl处理(MS)下棉花幼苗株高、干物重、根系形态、叶和根中抗氧化酶活性、丙二醛(MDA)以及可溶性糖含量的变化。结果表明, 150 mmol∙L−1 NaCl胁迫下, 棉苗株高比CK显著降低(P<0.05), 根系不发达, 干物重减少, 抗氧化酶[超氧化物歧化酶(SOD)、过氧化物酶(POD)、过氧化氢酶(CAT)、抗坏血酸过氧化物酶(APX)]活性及可溶性蛋白质含量显著降低(P<0.05), 而MDA含量增加(P<0.05); 10 μmol∙L−1褪黑素浸种后, 提高了盐胁迫下棉苗株高及生物量, 显著增加了根系总根长、侧根数目和主根直径(P<0.05), 并提高了根与叶中的SOD、POD、CAT和APX活性(P<0.05), 增加了可溶性糖含量(P<0.05), 降低了MDA含量(P<0.05); 在无NaCl胁迫下, 10 μmol∙L−1褪黑素浸种未能显著增加棉花株高, 但显著提高了主根直径和根中SOD、POD、CAT、APX活性(P<0.05), 增加了可溶性糖含量(P<0.05), 降低了MDA含量(P<0.05)。对19个代表性指标进行Spearman相关分析, 发现棉花幼苗总干重与株高、根系形态(总根长、主根长、根系平均直径、总侧根数目)、抗氧化酶活性(根与叶SOD、POD、CAT、APX)、可溶性糖含量均呈现显著正相关, 而与根和叶中MDA含量呈显著负相关。综合分析认为, 外源褪黑素可通过促进棉苗侧根发育和主根直径增粗以及增加抗氧化酶活性和可溶性糖含量, 减缓盐胁迫对棉花幼苗产生的伤害, 促进株高增加及干物质积累, 提高了棉苗的抗盐性。此外, 外源褪黑素还可促进正常条件下棉花幼苗发育, 这为褪黑素的开发利用及棉花栽培调控提供了理论依据。Abstract: Melatonin is an effective antioxidant that can promote the growth and development of plants under stress and alleviate stress-induced damage. The growth and development of cotton, an important cash crop in China, is severely impacted by salt stress. As such, here, we explored the regulatory effect of melatonin on the growth and development of cotton under salt stress by soaking ‘Guoxin Cotton No. 9’ seeds in different concentrations of melatonin (0, 0.1, 1, 10, 50, 100, 150 mmol∙L−1) under 150 mmol∙L−1 NaCl. We determined the root morphology (total root length, total surface area, total volume, number of lateral roots, root length, surface area and diameter), seedling height, and dry matter weight; thereafter, the most suitable melatonin concentration, 10 μmol∙L−1, was selected. Then, we measured and analyzed the activities of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX), as well as the contents of malondialdehyde (MDA) and soluble sugar in leaves and roots of cotton seedlings and the height, biomass, as well as root morphology indexes under 150 mmol∙L−1 NaCl, 10 μmol∙L−1 melatonin, and 150 mmol∙L−1 NaCl plus 10 μmol∙L−1 melatonin. The results revealed that under salt stress, the height of seedlings decreased, root systems were underdeveloped, dry matter weights decreased, the activities of antioxidant enzymes (SOD, POD, CAT, and APX) decreased, and soluble protein content decreased; however, the MDA content was found to increase when compared to normal, salt stress-free condition. After soaking the seeds in 10 μmol∙L−1 melatonin and 150 mmol∙L−1 NaCl, seedling heights and biomass, total root lengths, number of lateral roots, diameter of taproots, activities of SOD, POD, CAT, APX, and content of soluble sugar all increased, but MDA content decreased in roots and leaves. In the absence of salt stress, soaking the seeds in 10 μmol∙L−1 melatonin did not significantly increase the plant heights, but significantly increased taproot diameters, SOD, POD, CAT, and APX activities, as well as soluble sugar content in the cotton plant roots (P<0.05); however, significantly decreased MDA content (P<0.05). Spearman correlation analysis of 19 indices revealed that the total dry weight of seedlings was significantly and positively correlated with plant height, total root length, main root length, root mean diameter, total lateral root number, SOD, POD, CAT, and APX activities in roots and leaves, and soluble sugar content; however, there was a significant and negative correlation between total dry weight and MDA content in roots and leaves. With these comprehensive analyses, we show that exogenous melatonin could alleviate the damage caused by salt stress in cotton seedlings, promote an increase in plant height and dry matter accumulation, improve the resistance of cotton seedlings to salt stress by promoting lateral root development and thickening of main root, and increase the antioxidant enzyme activity and soluble sugar content. In addition, we reveal that exogenous melatonin can promote the development of cotton seedlings under salt stress-free condition; this provides a theoretical basis for the development and utilization of melatonin as well as the regulation of cotton cultivation.
-
Key words:
- Melatonin /
- Salt stress /
- Cotton seedling /
- Morphology /
- Antioxidant enzymes activity
-
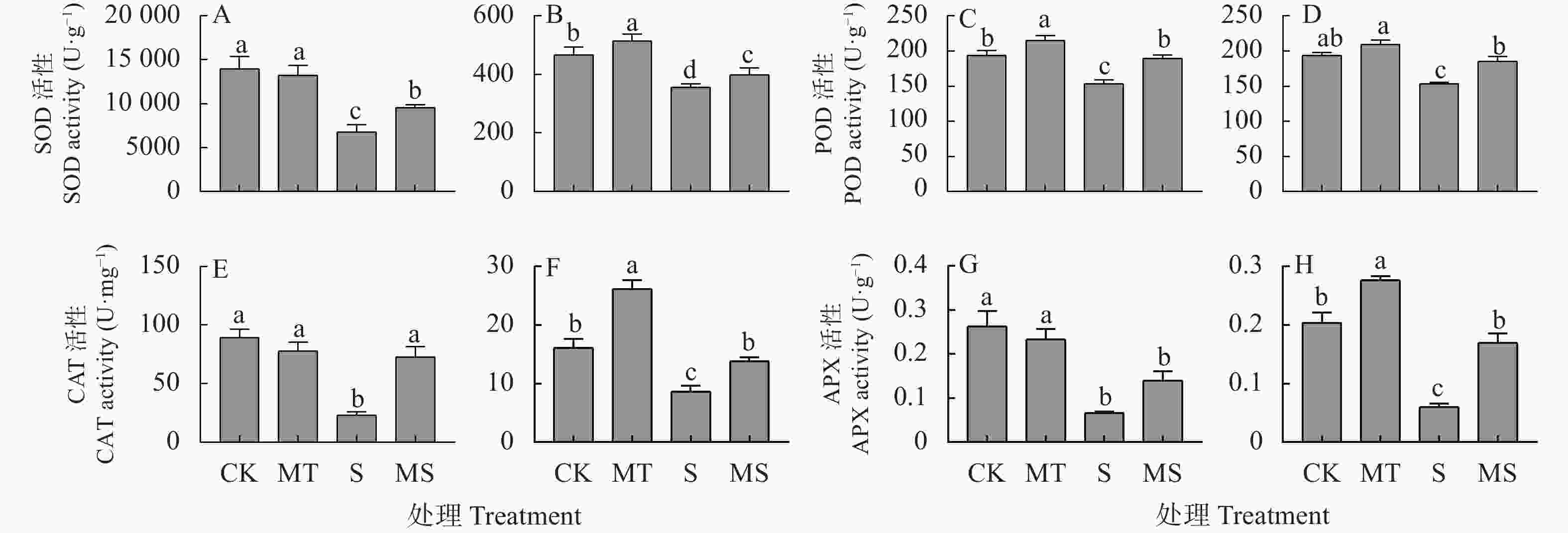
图 1 褪黑素对盐胁迫下棉花幼苗叶片(A, C, E, G)和根系(B, D, F, H)超氧化物歧化酶(SOD, A和B)、过氧化物酶(POD, C和D)、过氧化氢酶(CAT, E和F)和抗坏血酸过氧化物酶(APX, G和H)活性的影响
CK: 0 mmol∙L−1 NaCl+0 μmol∙L−1褪黑素; MT: 0 mmol∙L−1 NaCl+10 μmol∙L−1褪黑素; S: 150 mmol∙L−1 NaCl+0 μmol∙L−1褪黑素; MS: 150 mmol∙L−1 NaCl+10 μmol∙L−1褪黑素。不同小写字母表示不同处理间差异显著(P<0.05)。CK: 0 mmol∙L−1 NaCl+0 μmol∙L−1 melatonin; MT: 0 mmol∙L−1 NaCl+10 μmol∙L−1 melatonin; S: 150 mmol∙L−1 NaCl+0 μmol∙L−1 melatonin; MS : 150 mmol∙L−1 NaCl+10 μmol∙L−1 melatonin. Different lowercase letters mean significant differences among different treatments at P<0.05.
Figure 1. Effect of melatonin on activities of superoxide dismutase (SOD, A and B), peroxidase (POD, C and D), catalase (CAT, E and F) and ascorbate peroxidase (APX, G and H) of cotton seedling leaves (A, C, E, G) and roots (B, D, F, H) under salt stress
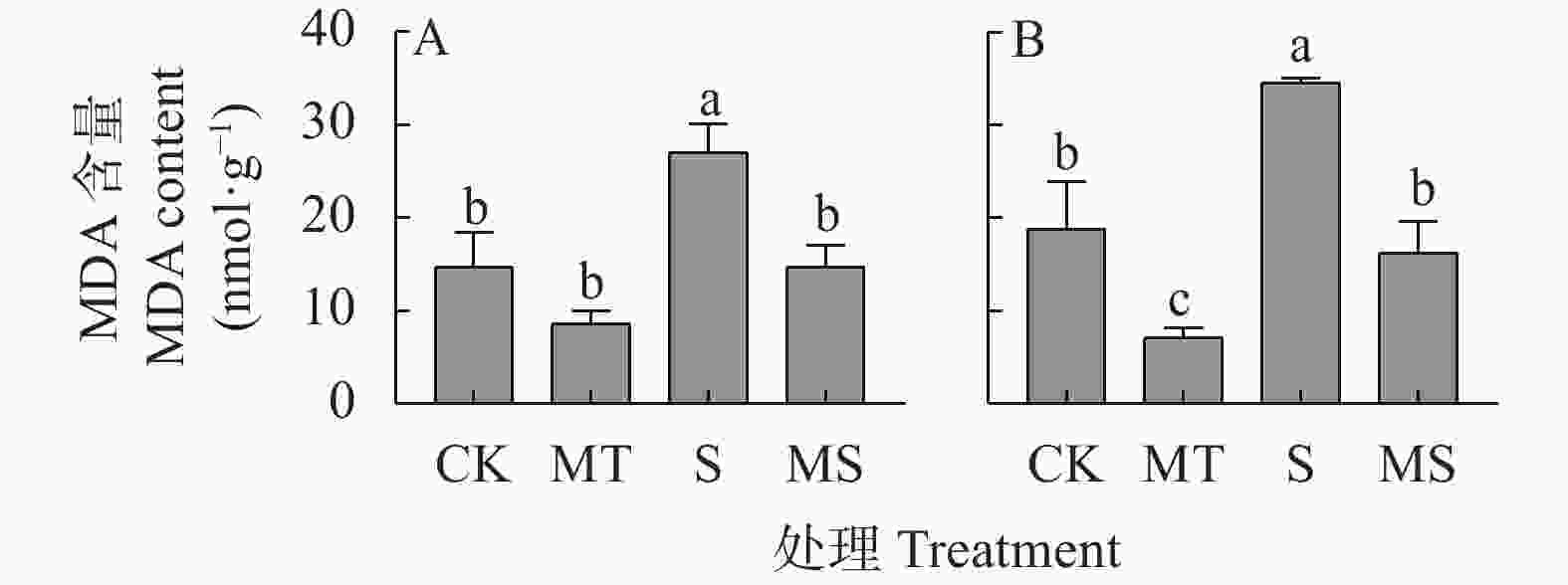
图 2 褪黑素对盐胁迫下棉花幼苗叶片(A)和根系(B)中丙二醛(MDA)含量的影响
CK: 0 mmol∙L−1 NaCl+0 μmol∙L−1褪黑素; MT: 0 mmol∙L−1 NaCl+10 μmol∙L−1褪黑素; S: 150 mmol∙L−1 NaCl+0 μmol∙L−1褪黑素; MS: 150 mmol∙L−1 NaCl+10 μmol∙L−1褪黑素。不同小写字母表示不同处理间差异显著(P<0.05)。CK: 0 mmol∙L−1 NaCl+0 μmol∙L−1 melatonin; MT: 0 mmol∙L−1 NaCl+10 μmol∙L−1 melatonin; S: 150 mmol∙L−1 NaCl+0 μmol∙L−1 melatonin; MS : 150 mmol∙L−1 NaCl+10 μmol∙L−1 melatonin. Different lowercase letters mean significant differences among different treatments at P<0.05.
Figure 2. Effects of melatonin on malondialdehyde (MDA) contents in leaves (A) and roots (B) of cotton seedlings under salt stress
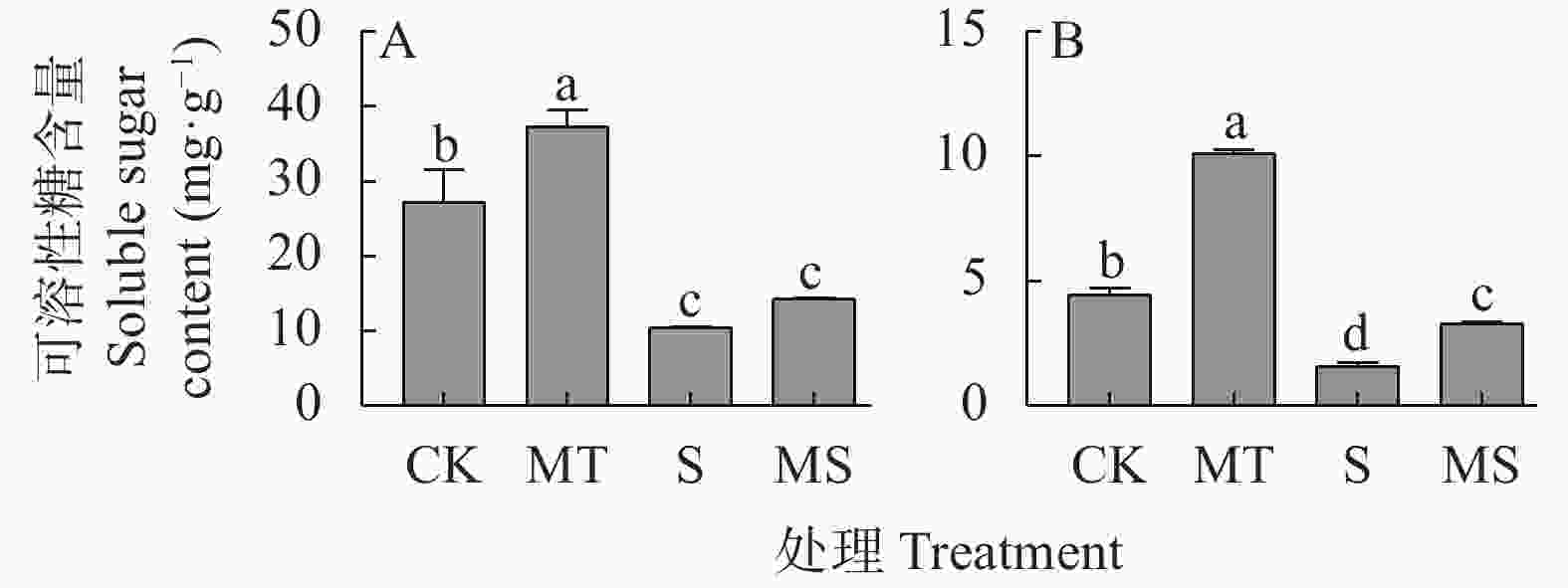
图 3 褪黑素对盐胁迫下棉花幼苗叶片(A)和根系(B)中可溶性糖含量的影响
CK: 0 mmol∙L−1 NaCl+0 μmol∙L−1褪黑素; MT: 0 mmol∙L−1 NaCl+10 μmol∙L−1褪黑素; S: 150 mmol∙L−1 NaCl+0 μmol∙L−1褪黑素; MS: 150 mmol∙L−1 NaCl+10 μmol∙L−1褪黑素。不同小写字母表示不同处理间差异显著(P<0.05)。CK: 0 mmol∙L−1 NaCl+0 μmol∙L−1 melatonin; MT: 0 mmol∙L−1 NaCl+10 μmol∙L−1 melatonin; S: 150 mmol∙L−1 NaCl+0 μmol∙L−1 melatonin; MS : 150 mmol∙L−1 NaCl+10 μmol∙L−1 melatonin. Different lowercase letters mean significant differences among different treatments at P<0.05.
Figure 3. Effect of melatonin on soluble sugar content in leaves (A) and roots (B) of cotton seedlings under salt stress
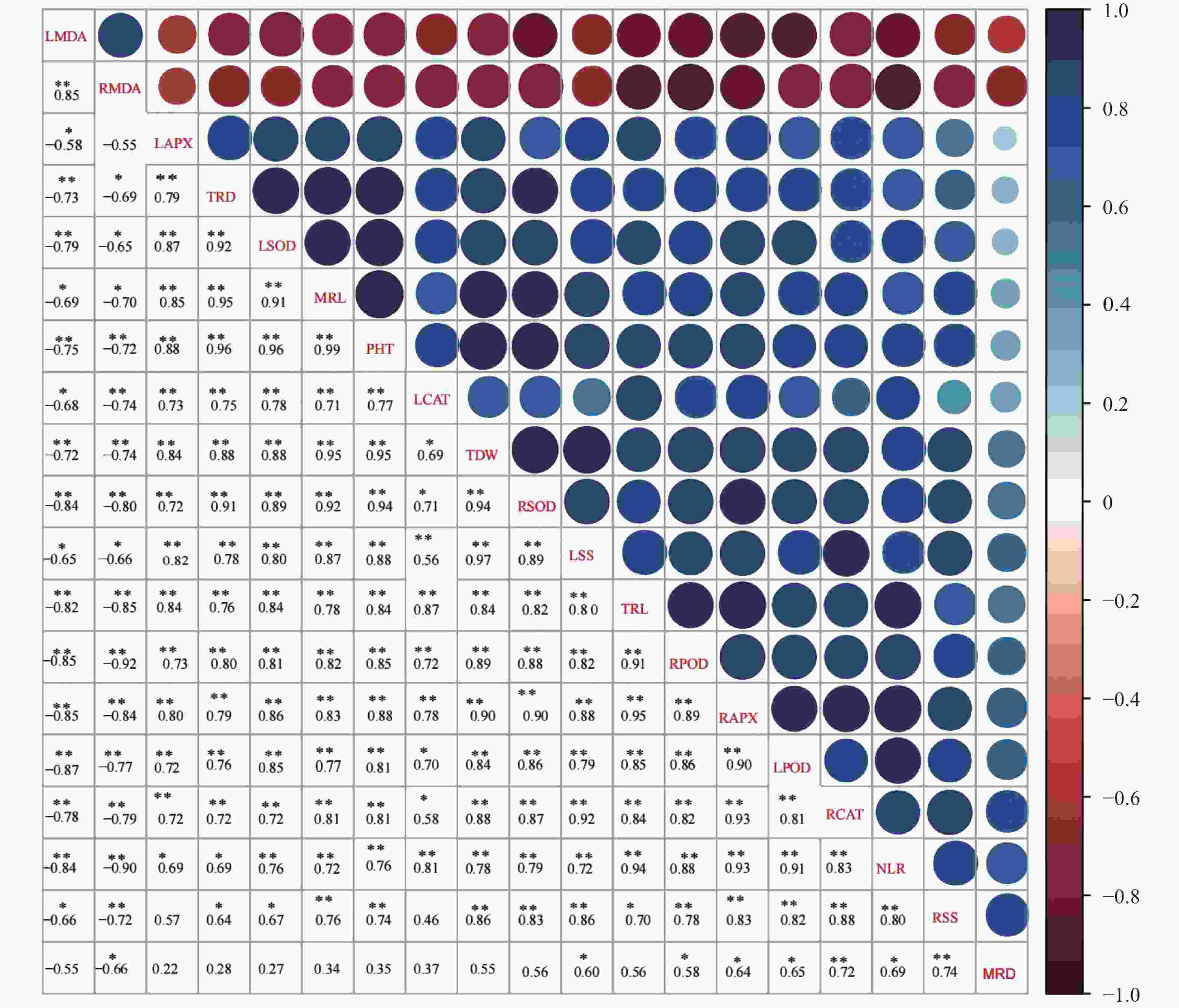
图 4 棉花幼苗19形态及生理指标的相关性分析
LMDA: 叶片丙二醛含量; RMDA: 根系丙二醛含量; LAPX: 叶片抗坏血酸过氧化物酶活性; TRD: 根系平均直径; LSOD: 叶片超氧化物歧化酶活性; MRL: 主根长; PHT: 株高; LCAT: 叶片过氧化氢酶活性; TDW: 总干重; RSOD: 根系超氧化物歧化酶活性; LSS: 叶片可溶性糖含量; TRL: 总根长; RPOD: 根系过氧化物酶活性; RAPX: 根系抗坏血酸过氧化物酶活性; LPOD: 叶片过氧化物酶活性; RCAT: 根系过氧化氢酶活性; NLR: 总侧根数目; RSS: 根系可溶性糖含量; MRD: 主根平均直径。星号表示显著的统计差异, *P≤0.05; **P≤0.01。LMDA: leaf malondialdehyde content; RMDA: roots malondialdehyde content; LAPX: leaves ascorbate peroxidase activity; TRD: mean root diameter; LSOD: leaves superoxide dismutase activity; MRL: taproot length; PHT: plant height; LCAT: leaves catalase activity; TDW: total dry weight; RSOD: roots superoxide dismutase activity; LSS: leaves soluble sugar content; TRL: total root length; RPOD: roots peroxidase activity; RAPX: roots ascorbate peroxidase activity; LPOD: leaves peroxidase activity; RCAT: roots catalase activity; NLR: total number of lateral roots; RSS: roots soluble sugar content; MRD: mean diameter of taproot. The asterisk indicates significant statistical difference. *: P≤0.05; **: P≤0.01.
Figure 4. Correlation analysis between 19 morphological and physiological indexes of cotton seedlings
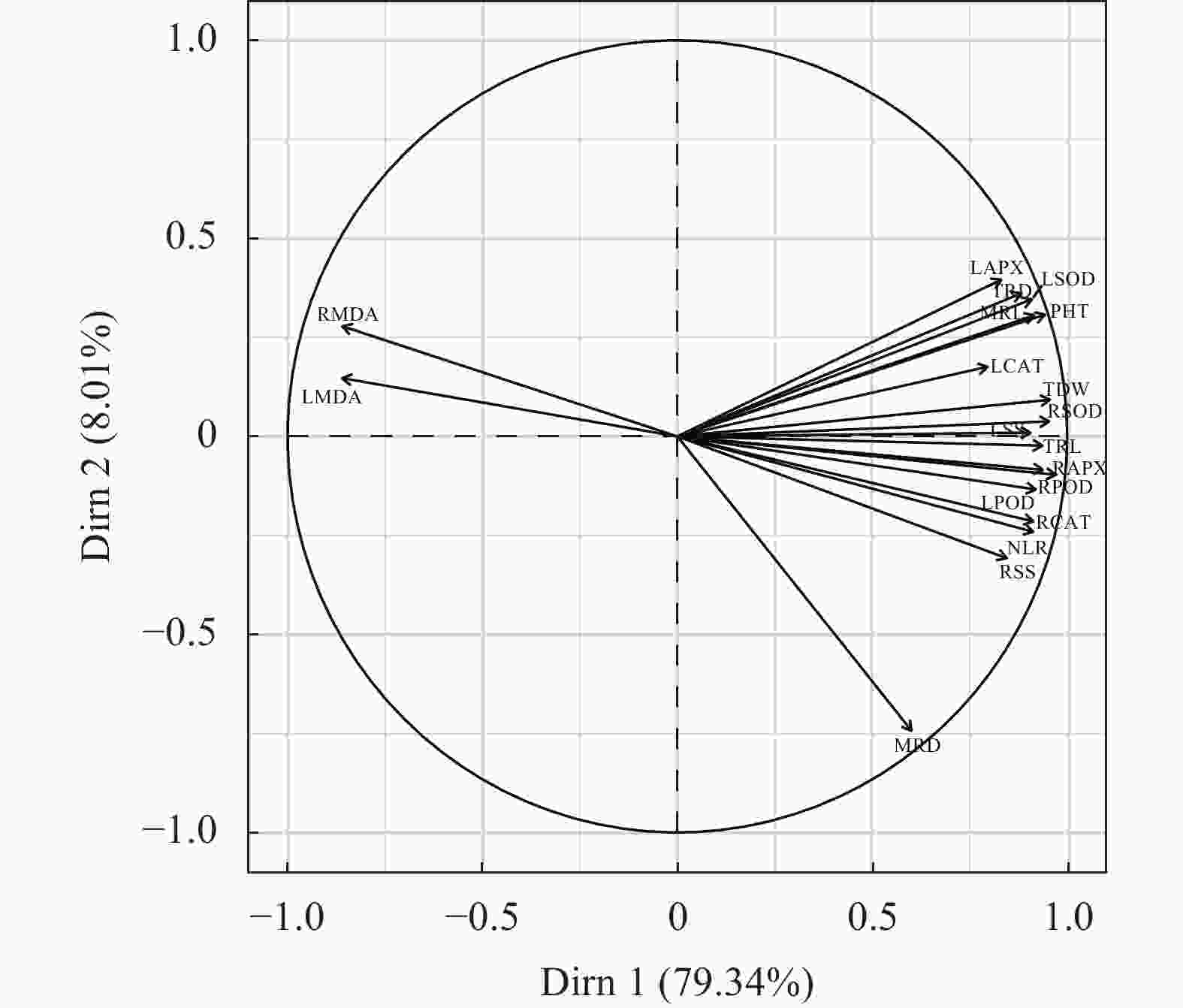
图 5 棉花幼苗19形态及生理指标的主成分分析
LMDA: 叶片丙二醛; RMDA: 根系丙二醛; LAPX: 叶片抗坏血酸过氧化物酶; TRD: 平均根系直径; LSOD: 叶片超氧化物歧化酶; MRL: 主根长; PHT: 株高; LCAT: 叶片过氧化氢酶; TDW: 总干重; RSOD: 根系超氧化物歧化酶; LSS: 叶片可溶性糖; TRL: 总根长; RPOD: 根系过氧化物酶; RAPX: 根系抗坏血酸过氧化物酶; LPOD: 叶片过氧化物酶; RCAT: 根系过氧化氢酶; NLR: 总侧根数目; RSS: 根系可溶性糖; MRD: 主根平均直径。星号表示显著的统计差异, * P≤0.05; ** P≤0.01。LMDA: leaf malondialdehyde; RMDA: root malondialdehyde; LAPX: leaves ascorbate peroxidase; TRD: mean root diameter; LSOD: leaf superoxide dismutase; MRL: taproot length; PHT: plant height; LCAT: leaf catalase; TDW: total dry weight ; RSOD: root superoxide dismutase; LSS: leaf soluble sugar; TRL: total root length; RPOD: root peroxidase; RAPX: root ascorbate peroxidase; LPOD: leaf peroxidase; RCAT: root catalase; NLR: total number of lateral roots; RSS: root soluble sugar; MRD: mean diameter of taproot. The asterisk indicates significant statistical difference. *: P≤0.05; **: P≤0.01.
Figure 5. Principal component analysis of 19 morphological and physiological indexes of cotton seedlings
表 1 不同浓度褪黑素(MT)对盐胁迫下棉花幼苗株高和干物质重量的影响
Table 1. Effects of melatonin (MT) under different concentrations on plant height and dry matter weight of cotton seedlings under salt stress
处理
Treatment株高
Plant height (mm)地上部干重
Shoot dry weight (g∙plant−1)地下部干重
Root dry weight (g∙plant−1)总干重
Total dry weight (g∙plant−1)CK 48.67±1.70a 0.94±0.12a 0.19±0.02a 1.13±0.14a S 22.62±0.46f 0.40±0.01c 0.09±0.01d 0.49±0.02c MT0.1 23.97±0.66ef 0.43±0.02c 0.12±0.01cd 0.54±0.03c MT1 26.84±1.43d 0.47±0.01bc 0.15±0.01bc 0.61±0.02bc MT10 32.78±0.82b 0.56±0.01b 0.17±0.01ab 0.73±0.01b MT50 29.08±1.04c 0.51±0.01bc 0.14±0.01bc 0.65±0.02bc MT100 25.98±0.48de 0.41±0.01c 0.12±0.01cd 0.53±0.02c MT150 22.94±1.16f 0.38±0.01c 0.10±0.01d 0.49±0.02c CK: 0 mmol∙L−1 NaCl+0 μmol∙L−1褪黑素; S: 150 mmol∙L−1 NaCl+0 μmol∙L−1褪黑素; MT0.1: 150 mmol∙L−1 NaCl+0.1 μmol∙L−1褪黑素; MT1: 150 mmol∙L−1 NaCl+1 μmol∙L−1褪黑素; MT10: 150 mmol∙L−1 NaCl+10 μmol∙L−1褪黑素; MT50: 150 mmol∙L−1 NaCl+50 μmol∙L−1褪黑素; MT100: 150 mmol∙L−1 NaCl+100 μmol∙L−1褪黑素; MT150: 150 mmol∙L−1 NaCl+150 μmol∙L−1褪黑素。同列不同小写字母表示不同褪黑素浓度间差异显著(P<0.05)。CK: 0 mmol∙L−1 NaCl+0 μmol∙L−1 melatonin; S: 150 mmol∙L−1 NaCl+0 μmol∙L−1 melatonin; MT0.1: 150 mmol∙L−1 NaCl+0.1 μmol∙L−1 melatonin; MT1: 150 mmol∙L−1 NaCl+1 μmol∙L−1 melatonin; MT10: 150 mmol∙L−1 NaCl+10 μmol∙L−1 melatonin; MT50: 150 mmol∙L−1 NaCl+50 μmol∙L−1 melatonin; MT100: 150 mmol∙L−1 NaCl+100 μmol∙L−1 melatonin; MT150: 150 mmol∙L−1 NaCl+150 μmol∙L−1 melatonin. Different lowercase letters in the same column mean significant differences among different melatonin concentrations at P<0.05. 表 2 不同浓度褪黑素(MT)对棉花幼苗根系形态的影响
Table 2. Effects of melatonin (MT) under different concentrations on root morphology of cotton seedlings
处理
Treatment总根长
Total length (cm)总投影面积
Total projected area (cm2)总表面积
Total surface area (cm2)平均直径
Average diameter (mm)总体积
Total volume (cm3)CK 845.80±9.85a 53.84±2.43a 157.15±6.10a 0.64±0.03a 2.13±0.08a S 505.93±2.15d 24.09±1.40e 84.68±2.42c 0.48±0.03b 0.92±0.10c MT0.1 663.44±83.18bc 31.82±1.28d 104.47±19.15bc 0.49±0.07b 1.37±0.32b MT1 721.33±157.26bc 37.80±3.79c 118.95±26.44b 0.54±0.11ab 1.50±0.20b MT10 794.95±3.80ab 46.62±1.39b 131.58±1.56ab 0.59±0.02ab 1.74±0.17b MT50 764.60±22.34ab 43.83±2.86b 119.78±6.80b 0.57±0.04ab 1.63±0.04b MT100 705.01±5.13bc 38.54±0.54c 111.09±4.93b 0.55±0.01ab 1.44±0.08b MT150 604.15±4.31cd 32.23±1.07d 106.49±3.33bc 0.53±0.01ab 0.93±0.11c CK: 0 mmol∙L−1 NaCl+0 μmol∙L−1褪黑素; S: 150 mmol∙L−1 NaCl+0 μmol∙L−1褪黑素; MT0.1: 150 mmol∙L−1 NaCl+0.1 μmol∙L−1褪黑素; MT1: 150 mmol∙L−1 NaCl+1 μmol∙L−1褪黑素; MT10: 150 mmol∙L−1 NaCl+10 μmol∙L−1褪黑素; MT50: 150 mmol∙L−1 NaCl+50 μmol∙L−1褪黑素; MT100: 150 mmol∙L−1 NaCl+100 μmol∙L−1褪黑素; MT150: 150 mmol∙L−1 NaCl+150 μmol∙L−1褪黑素。同列不同小写字母表示不同褪黑素浓度间差异显著(P<0.05)。CK: 0 mmol∙L−1 NaCl+0 μmol∙L−1 melatonin; S: 150 mmol∙L−1 NaCl+0 μmol∙L−1 melatonin; MT0.1: 150 mmol∙L−1 NaCl+0.1 μmol∙L−1 melatonin; MT1: 150 mmol∙L−1 NaCl+1 μmol∙L−1 melatonin; MT10: 150 mmol∙L−1 NaCl+10 μmol∙L−1 melatonin; MT50: 150 mmol∙L−1 NaCl+50 μmol∙L−1 melatonin; MT100: 150 mmol∙L−1 NaCl+100 μmol∙L−1 melatonin; MT150: 150 mmol∙L−1 NaCl+150 μmol∙L−1 melatonin. Different lowercase letters in the same column mean significant differences among different melatonin concentrations at P<0.05. 表 3 褪黑素对盐胁迫下棉花幼苗株高和干物质重量的影响
Table 3. Effects of melatonin on plant height and dry matter weight of cotton seedlings under salt stress
处理
Treatment株高
Plant height (mm)地上部干重
Shoot dry weight (g∙plant−1)地下部干重
Root dry weight (g∙plant−1)总干重
Total dry weight (g∙plant−1)CK 46.78±2.28a 0.83±0.05b 0.15±0.01ab 0.98±0.05b MT 47.89±0.57a 1.00±0.03a 0.18±0.01a 1.19±0.04a S 23.89±0.16c 0.39±0.03d 0.07±0.01c 0.45±0.04d MS 30.22±0.42b 0.49±0.01c 0.13±0.02b 0.62±0.01c CK: 0 mmol∙L−1 NaCl+0 μmol∙L−1褪黑素; MT: 0 mmol∙L−1 NaCl+10 μmol∙L−1褪黑素; S: 150 mmol∙L−1 NaCl+0 μmol∙L−1褪黑素; MS: 150 mmol∙L−1 NaCl+10 μmol∙L−1褪黑素。同列不同小写字母表示不同处理间差异显著(P<0.05)。CK: 0 mmol∙L−1 NaCl+0 μmol∙L−1 melatonin; MT: 0 mmol∙L−1 NaCl+10 μmol∙L−1 melatonin; S : 150 mmol∙L−1 NaCl+0 μmol∙L−1 melatonin; MS : 150 mmol∙L−1 NaCl+10 μmol∙L−1 melatonin. Different lowercase letters in the same column mean significant differences among different treatments at P<0.05. 表 4 褪黑素对盐胁迫下棉花幼苗根系形态的影响
Table 4. Effects of melatonin on root morphology of cotton seedlings under salt stress
特征 Character CK MT S MS 总长 Total length (cm) 852.77±34.18ab 916.93±28.25a 509.10±17.01c 798.96±38.45b 总投影面积 Total projected area (cm2) 59.97±2.93a 63.25±2.32a 23.12±2.09c 41.64±1.07b 总表面积 Total surface area (cm2) 156.50±19.08ab 170.60±12.84a 85.41±8.81c 127.74±4.45b 平均直径 Average diameter (mm) 0.71±0.06a 0.69±0.03a 0.46±0.03b 0.52±0.01b 总体积 Total volume (cm3) 2.18±0.56ab 2.55±0.92a 0.97±0.11b 1.47±0.28ab 一级侧根数 Number of first-order lateral roots 54.3±1.7b 67.0±3.3a 41.3±1.3c 56.3±2.9b 二级侧根数 Number of secondary lateral roots 163.7±3.1b 193.3±12.0a 103.7±4.9c 171.3±3.1b 总侧根数目 Number of lateral roots 218.0±4.3b 260.3±13.5a 145.0±4.3c 227.7±0.9b 主根长 Main root length (cm) 19.09±0.54a 19.54±0.19a 14.95±0.04b 15.61±0.36b 主根平均直径 Average diameter of taproot (mm) 1.42±0.08c 1.96±0.07a 1.43±0.02c 1.70±0.14b 主根表面积 Surface area of taproot (cm2) 8.13±0.45c 14.76±1.63a 6.95±1.19c 10.98±0.28b 主根体积 Volume of taproot (cm3) 2.21±0.15ab 2.72±0.32a 1.27±0.26c 1.79±0.11bc CK: 0 mmol∙L−1 NaCl+0 μmol∙L−1褪黑素; MT: 0 mmol∙L−1 NaCl+10 μmol∙L−1褪黑素; S: 150 mmol∙L−1 NaCl+0 μmol∙L−1褪黑素; MS: 150 mmol∙L−1 NaCl+10 μmol∙L−1褪黑素。同行不同小写字母表示不同处理间差异显著(P<0.05)。CK: 0 mmol∙L−1 NaCl+0 μmol∙L−1 melatonin; MT: 0 mmol∙L−1 NaCl+10 μmol∙L−1 melatonin; S: 150 mmol∙L−1 NaCl+0 μmol∙L−1 melatonin; MS : 150 mmol∙L−1 NaCl+10 μmol∙L−1 melatonin. Different lowercase letters in the same row mean significant differences among different treatments at P<0.05. -
[1] 李建国, 濮励杰, 朱明, 等. 土壤盐渍化研究现状及未来研究热点[J]. 地理学报, 2012, 67(9): 1233−1245 doi: 10.11821/xb201209008LI J G, PU L J, ZHU M, et al. The present situation and hot issues in the salt-affected soil research[J]. Acta Geographica Sinica, 2012, 67(9): 1233−1245 doi: 10.11821/xb201209008 [2] 李彦, 张英鹏, 孙明, 等. 盐分胁迫对植物的影响及植物耐盐机理研究进展[J]. 中国农学通报, 2008, 24(1): 258−265LI Y, ZHANG Y P, SUN M, et al. Research advance in the effects of salt stress on plant and the mechanism of plant resistance[J]. Chinese Agricultural Science Bulletin, 2008, 24(1): 258−265 [3] 王康君, 樊继伟, 陈凤, 等. 植物对盐胁迫的响应及耐盐调控的研究进展[J]. 江西农业学报, 2018, 30(12): 31−40WANG K J, FAN J W, CHEN F, et al. Research advances in response of plants to salt stress and regulation of salinity tolerance[J]. Acta Agriculturae Jiangxi, 2018, 30(12): 31−40 [4] 辛承松, 董合忠, 唐薇, 等. 棉花盐害与耐盐性的生理和分子机理研究进展[J]. 棉花学报, 2005, 17(5): 309−313 doi: 10.3969/j.issn.1002-7807.2005.05.011XIN C S, DONG H Z, TANG W, et al. Physiological and molecular mechanisms of salt injury and salt tolerance in cotton[J]. Acta Gossypii Sinica, 2005, 17(5): 309−313 doi: 10.3969/j.issn.1002-7807.2005.05.011 [5] ZHANG N, ZHAO B, ZHANG H J, et al. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.)[J]. Journal of Pineal Research, 2013, 54(1): 15−23 doi: 10.1111/j.1600-079X.2012.01015.x [6] 马玉花. 植物耐盐分子机理研究进展[J]. 湖北农业科学, 2013, 52(2): 255−257 doi: 10.3969/j.issn.0439-8114.2013.02.002MA Y H. Research progress on the molecular mechanism of plant salt-tolerance[J]. Hubei Agricultural Sciences, 2013, 52(2): 255−257 doi: 10.3969/j.issn.0439-8114.2013.02.002 [7] NOREEN Z, ASHRAF M. Assessment of variation in antioxidative defense system in salt-treated pea (Pisum sativum) cultivars and its putative use as salinity tolerance markers[J]. Journal of Plant Physiology, 2009, 166(16): 1764−1774 doi: 10.1016/j.jplph.2009.05.005 [8] AHMAD M S A, ALI Q, ASHRAF M, et al. Involvement of polyamines, abscisic acid and anti-oxidative enzymes in adaptation of Blue Panicgrass (Panicum antidotale Retz.) to saline environments[J]. Environmental and Experimental Botany, 2009, 66(3): 409−417 doi: 10.1016/j.envexpbot.2009.02.011 [9] 朱金方, 刘京涛, 陆兆华, 等. 盐胁迫对中国柽柳幼苗生理特性的影响[J]. 生态学报, 2015, 35(15): 5140−5146ZHU J F, LIU J T, LU Z H, et al. Effects of salt stress on physiological characteristics of Tamarix chinensis Lour. seedlings[J]. Acta Ecologica Sinica, 2015, 35(15): 5140−5146 [10] LERNER A B, CASE J D, TAKAHASHI Y, et al. Isolation of melatonin, the pineal gland factor that lightens melanocytes1[J]. Journal of the American Chemical Society, 1958, 80(10): 2587 [11] NAWAZ M A, HUANG Y, BIE Z L, et al. Melatonin: current status and future perspectives in plant science[J]. Frontiers in Plant Science, 2015, 6: 1230 [12] RAMAKRISHNA A, GIRIDHAR P, SANKAR K U, et al. Melatonin and serotonin profiles in beans of Coffea species[J]. Journal of Pineal Research, 2012, 52(4): 470−476 doi: 10.1111/j.1600-079X.2011.00964.x [13] POSMYK M M, BAŁABUSTA M, WIECZOREK M, et al. Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling stress[J]. Journal of Pineal Research, 2009, 46(2): 214−223 doi: 10.1111/j.1600-079X.2008.00652.x [14] 陈莉, 刘连涛, 马彤彤, 等. 褪黑素对盐胁迫下棉花种子抗氧化酶活性及萌发的影响[J]. 棉花学报, 2019, 31(5): 438−447 doi: 10.11963/1002-7807.cllcd.20190905CHEN L, LIU L T, MA T T, et al. Effects of melatonin on the antioxidant enzyme activities and seed germination of cotton (Gossypium hirsutum L.) under salt-stress conditions[J]. Cotton Science, 2019, 31(5): 438−447 doi: 10.11963/1002-7807.cllcd.20190905 [15] CASTAÑARES J L, BOUZO C A. Effect of exogenous melatonin on seed germination and seedling growth in melon (Cucumis melo L.) under salt stress[J]. Horticultural Plant Journal, 2019, 5(2): 79−87 doi: 10.1016/j.hpj.2019.01.002 [16] ZENG L, CAI J S, LI J J, et al. Exogenous application of a low concentration of melatonin enhances salt tolerance in rapeseed (Brassica napus L.) seedlings[J]. Journal of Integrative Agriculture, 2018, 17(2): 328−335 doi: 10.1016/S2095-3119(17)61757-X [17] CHEN Z P, XIE Y J, GU Q, et al. The AtrbohF-dependent regulation of ROS signaling is required for melatonin-induced salinity tolerance in Arabidopsis[J]. Free Radical Biology and Medicine, 2017, 108: 465−477 doi: 10.1016/j.freeradbiomed.2017.04.009 [18] YE J, YANG W J, LI Y L, et al. Seed pre-soaking with melatonin improves wheat yield by delaying leaf senescence and promoting root development[J]. Agronomy, 2020, 10(1): 84 doi: 10.3390/agronomy10010084 [19] PARK S, BACK K. Melatonin promotes seminal root elongation and root growth in transgenic rice after germination[J]. Journal of Pineal Research, 2012, 53(4): 385−389 doi: 10.1111/j.1600-079X.2012.01008.x [20] 习林杰. 盐胁迫下外源褪黑素对生菜幼苗根系的影响[D]. 杨凌: 西北农林科技大学, 2019: 7–9XI L J. Effects of exogenous melatonin on root system of lettuce seedlings under salt stress[D]. Yangling: Northwest A&F University, 2019: 7–9 [21] 张国伟, 路海玲, 张雷, 等. 棉花萌发期和苗期耐盐性评价及耐盐指标筛选[J]. 应用生态学报, 2011, 22(8): 2045−2053ZHANG G W, LU H L, ZHANG L, et al. Salt tolerance evaluation of cotton (Gossypium hirsutum) at its germinating and seedling stages and selection of related indices[J]. Chinese Journal of Applied Ecology, 2011, 22(8): 2045−2053 [22] 孙小芳, 刘友良, 陈沁. 棉花耐盐性研究进展[J]. 棉花学报, 1998, 10(3): 7−13SUN X F, LIU Y L, CHEN Q. Recent progresses in studies on salinity tolerence in cotton[J]. Acta Gossypii Sinica, 1998, 10(3): 7−13 [23] JIANG D, LU B, LIU L T, et al. Exogenous melatonin improves salt stress adaptation of cotton seedlings by regulating active oxygen metabolism[J]. PeerJ, 2020, 8: e10486 doi: 10.7717/peerj.10486 [24] MU X W, YAN M W, DE Z C, et al. Changes of free radicals and digestive enzymes in saliva in cases with deficiency in spleen-yin syndrome[J]. Journal of Biomedical Research, 2010, 24(3): 250−255 doi: 10.1016/S1674-8301(10)60035-8 [25] HONG X L, YU X, LING L C, et al. Cerebroside C increases tolerance to chilling injury and alters lipid composition in wheat roots[J]. PLOS ONE, 2013, 8(9): e73380. doi: 10.1371/journal.pone.0073380 [26] ZHANG J Q, MING S, ZHU C C, et al. 3-nitropropionic acid induces ovarian oxidative stress and impairs follicle in mouse[J]. PLoS ONE, 2014, 9(2): e86589. doi: 10.1371/journal.pone.0086589 [27] 李合生. 植物生理生化实验原理和技术[M]. 北京: 高等教育出版社, 2000: 195–197LI H S. Principles and Techniques of Plant Physiological and Biochemical Experiments[M]. Beijing: Higher Education Press, 2000: 195–197 [28] 吴灏. 旱涝胁迫对棉花生长和产量的影响及模拟[D]. 武汉: 武汉大学, 2018: 6–8WU H. Simulation for the growth and yield of cotton in response to drought and waterlogging[D]. Wuhan: Wuhan University, 2018: 6–8 [29] 严青青, 张巨松, 徐海江, 等. 盐碱胁迫对海岛棉幼苗生物量分配和根系形态的影响[J]. 生态学报, 2019, 39(20): 7632−7640YAN Q Q, ZHANG J S, XU H J, et al. Effect of saline-alkali stress on biomass allocation and root morphology of sea island cotton seedlings[J]. Acta Ecologica Sinica, 2019, 39(20): 7632−7640 [30] 白燕丹. 褪黑素浸种对干旱胁迫下棉花种子萌发和幼苗生长的影响[D]. 保定: 河北农业大学, 2021: 38–40BAI Y D. Effects of seed soaking with melatonin on seed germination and seedling growth under drought stress in cotton[D]. Baoding: Hebei Agricultural University, 2021: 38–40 [31] 杨新元. 外源褪黑素对干旱胁迫下向日葵幼苗生长、光合及抗氧化系统的影响[J]. 华北农学报, 2019, 34(4): 113−121 doi: 10.7668/hbnxb.201751697YANG X Y. Effects of exogenous melatonin on growth, photosynthesis and antioxidant system of sunflower seedling under drought stress[J]. Acta Agriculturae Boreali-Sinica, 2019, 34(4): 113−121 doi: 10.7668/hbnxb.201751697 [32] 吕晓滨. 棉花高产优质栽培技术[M]. 呼和浩特: 内蒙古人民出版社, 2009: 32–35LYU X B. Cultivation Technology of Cotton with High Yield and Good Quality[M]. Hohhot: Inner Mongolia People’s Press, 2009: 32–35 [33] GAO K, CHEN F J, YUAN L X, et al. Cell production and expansion in the primary root of maize in response to low-nitrogen stress[J]. Journal of Integrative Agriculture, 2014, 13(11): 2508−2517 doi: 10.1016/S2095-3119(13)60523-7 [34] 郭建荣, 郑聪聪, 李艳迪, 等. NaCl处理对真盐生植物盐地碱蓬根系特征及活力的影响[J]. 植物生理学报, 2017, 53(1): 63−70GUO J R, ZHENG C C, LI Y D, et al. Effects of NaCl treatment on root system characteristics and activity of the euhalophyte Suaeda salsa[J]. Plant Physiology Journal, 2017, 53(1): 63−70 [35] 马旭辉, 陈茹梅, 柳小庆, 等. 褪黑素对玉米幼苗根系发育和抗旱性的影响[J]. 生物技术通报, 2021, 37(2): 1−14MA X H, CHEN R M, LIU X Q, et al. Effects of melatonin on root growth and drought tolerance of maize seedlings[J]. Biotechnology Bulletin, 2021, 37(2): 1−14 [36] WANG Q N, AN B, WEI Y X, et al. Melatonin regulates root meristem by repressing auxin synthesis and polar auxin transport in Arabidopsis[J]. Frontiers in Plant Science, 2016, 7: 1882 doi: 10.3389/fpls.2016.01882 [37] 李振庆. 棉花幼苗低盐驯化后对高盐胁迫的生理响应[D]. 北京: 中国农业科学院, 2017: 12–14LI Z Q. Physiological responses of cotton to salt stress after adaptation under low salinity in seeding stage[D]. Beijing: Chinese Academy of Agricultural Sciences, 2017: 12–14 [38] 李璇, 岳红, 王升, 等. 影响植物抗氧化酶活性的因素及其研究热点和现状[J]. 中国中药杂志, 2013, 38(7): 973−978LI X, YUE H, WANG S, et al. Research of different effects on activity of plant antioxidant enzymes[J]. China Journal of Chinese Materia Medica, 2013, 38(7): 973−978 [39] 邵怡若, 许建新, 薛立, 等. 低温胁迫时间对4种幼苗生理生化及光合特性的影响[J]. 生态学报, 2013, 33(14): 4237−4247 doi: 10.5846/stxb201301150100SHAO Y R, XU J X, XUE L, et al. Effects of low temperature stress on physiological-biochemical indexes and photosynthetic characteristics of seedlings of four plant species[J]. Acta Ecologica Sinica, 2013, 33(14): 4237−4247 doi: 10.5846/stxb201301150100 [40] 辛承松, 唐薇, 王洪征, 等. 鲁棉14幼苗生长对氯化钠胁迫的反应及微量元素、激素处理的效应[J]. 棉花学报, 2002, 14(2): 108−112 doi: 10.3969/j.issn.1002-7807.2002.02.010XIN C S, TANG W, WANG H Z, et al. Responses of seedlings growth of Lumian 14 to NaCl stress and effects of treatments with microelement and hormone[J]. Cotton Science, 2002, 14(2): 108−112 doi: 10.3969/j.issn.1002-7807.2002.02.010 [41] 崔桂宾. 干旱和PEG胁迫下小麦幼苗对褪黑素处理的生理响应及其蛋白质组学分析[D]. 杨凌: 西北农林科技大学, 2019: 61–67CUI G B. Physiological responses and proteomic analysis of melatonin treatment in wheat seedlings under drought and PEG stress[D]. Yangling: Northwest A&F University, 2019: 61–67 [42] ZHANG H J, ZHANG N, YANG R C, et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA₄ interaction in cucumber (Cucumis sativus L.)[J]. Journal of Pineal Research, 2014, 57(3): 269−279 doi: 10.1111/jpi.12167 [43] MA Q X, ZHANG T, ZHANG P, et al. Melatonin attenuates postharvest physiological deterioration of cassava storage roots[J]. Journal of Pineal Research, 2016, 60(4): 424−434 doi: 10.1111/jpi.12325 [44] MOHAMED I A A, SHALBY N, EL-BADRI A M A, et al. Stomata and xylem vessels traits improved by melatonin application contribute to enhancing salt tolerance and fatty acid composition of Brassica napus L. plants[J]. Agronomy, 2020, 10(8): 1186 doi: 10.3390/agronomy10081186 [45] PELAGIO-FLORES R, MUÑOZ-PARRA E, ORTIZ-CASTRO R, et al. Melatonin regulates Arabidopsis root system architecture likely acting independently of auxin signaling[J]. Journal of Pineal Research, 2012, 53(3): 279−288 doi: 10.1111/j.1600-079X.2012.00996.x -





 下载:
下载: