Response mechanism of soil fungal community in farmland during a period of chromium stress
-
摘要: 为探究农田土壤中真菌群落在时间序列对铬胁迫的响应机制及演变特征, 本研究以种植‘晋谷21号’谷子的土壤 (偏碱性的褐土) 为试验材料, 并在铬处理 (1 mmol∙L−1 Cr6+) 前 (CK) 及处理后6 h (Cr_6 h) 与6 d (Cr_6 d) 分别取样, 通过高通量测序及数据统计分析, 探究农田土壤中真菌群落在铬胁迫时间序列上的响应机制、群落构建驱动机制及功能预测分析。结果表明, 铬胁迫时间序列上, 农田土壤中真菌群落的组成结构差异显著, α多样性中的Shannon指数在Cr_6 d阶段显著下降 (CK为4.17、 Cr_6 h为3.81, Cr_6 d为3.23); 土壤中真菌群落的构建主要由随机过程主导 (beta NTI: −0.16, −0.71, −0.23); 且随铬胁迫时间增长, 群落分布更加广泛(迁移率m: CK为0.066、Cr_6 h为0.132、Cr_6 d为0.163), 即迁移率增大, 种间关系以共生为主, 赤霉菌属、镰刀菌属、金孢子菌属等是共生网络中的关键菌种; 功能预测分析表明铬胁迫时间序列上土壤中真菌群落以病原、腐生营养型为主, 高丰度的镰刀菌属等表明土壤中可能存在病原菌污染。研究结果表明在铬胁迫时间序列上, 土壤中真菌群落组分及组成结构变化显著, 群落构建由随机过程主导且群落所受扩散限制减小, 共生网络的种间关系复杂化。本研究通过铬胁迫处理农田土壤, 模拟土壤微生物群落对胁迫的应激与反应过程, 对重金属污染土壤的治理修复及推动可持续农业发展具有重要意义。Abstract: Heavy metal chromium (Cr) is one of China’s main soil pollutants and poses a great threat to its agricultural soils, especially in the Shanxi Province, where the soil Cr content is higher than the national average. A new millet (Setaria italica) variety, ‘Jingu 21’, has many advantages such as high quality, high yield and disease resistance. To investigate the changes and response mechanisms of fungal communities in agricultural soils during a period of Cr stress, we used soil (alkaline brown soil) planted with ‘Jingu 21’ as this study’s experimental material. Soil samples were taken before the introduction of Cr (CK), as well as 6 h (Cr_6 h) and 6 d (Cr_6 d) after 1 mmol L−1 of Cr6+ was introduced to the soil. High-throughput sequencing and statistical analysis of the data were used to investigate the response mechanism, the soil fungal community establishment, and the functional prediction of fungal communities in ‘Jingu 21’ soils during the period of Cr stress. The spatial and temporal distribution patterns of soil fungal communities were investigated using the non-metric multidimensional scale analysis, the soil fungal community establishment driving mechanism was investigated by constructing an interspecific symbiotic network diagram and a neutral community model (NCM), and the changes in soil fungal community function were investigated using FUNGuild. The results revealed that the composition and structure of soil fungal communities differed significantly at the phylum and genus levels during the period of Cr stress, and the Shannon diversity index of the community decreased significantly (P<0.05) at the Cr_6 d stage (4.17 for CK, 3.81 for Cr_6 h, and 3.23 for Cr_6 d). The spatial and temporal distribution patterns of fungal communities were similar within the same Cr stress period and differed significantly across these periods. The fungal community establishment was dominated by stochastic process (beta NTI: −0.16 for CK, −0.71 for Cr_6 h, and −0.23 for Cr_6 d). The interspecific symbiotic network analysis revealed that the fungal species were mostly positively correlated with each other; the interspecific symbiotic network of the Cr_6 d stage had a higher number of edges, average degree, and average path length than those of the CK and Cr_6 h stages, indicating that the community was more stable in the Cr_6 d stage than in the CK and Cr_6 h stage. Gibberella, Fusarium, and Chrysosporium were the key genera in the network diagram. The NCM quantified the stochastic processes further indicated that the soil fungal community was widely distributed (migration rate m: 0.066 for CK, 0.132 for Cr_6 h, and 0.163 for Cr_6 d). The FUNGuild function prediction showed that the soil fungal community was dominated by pathogenic and saprophytic trophic types. In addition, the abundance of sensitive bacteria, such as Mortierella and Gibberella, decreased, and the abundance of resistant bacteria, such as Fusarium, increased, indicating that Cr stress may affected the abundance of sensitive and resistant fungi in the soil, with the highest abundance of Fusarium indicating possible soil contamination with pathogenic bacteria. Ultimately, the results of this study revealed that the fungal community in soil planted with ‘Jingu 21’ changed significantly during a period of Cr stress; the soil fungal community establishment was dominated by stochastic processes; the diffusion restrictions of the community gradually decreased; and the interspecific relationships were complex and primarily symbiotic. In conclusion, herein, we simulated the stress response of soil microbial communities to Cr stress by treating agricultural soils with Cr6+. In addition, we demonstrated the response mechanism of soil fungal communities during a period of Cr stress, which is an important consideration for the treatment and remediation of heavy metal-contaminated soils and the promotion of sustainable agricultural development.
-
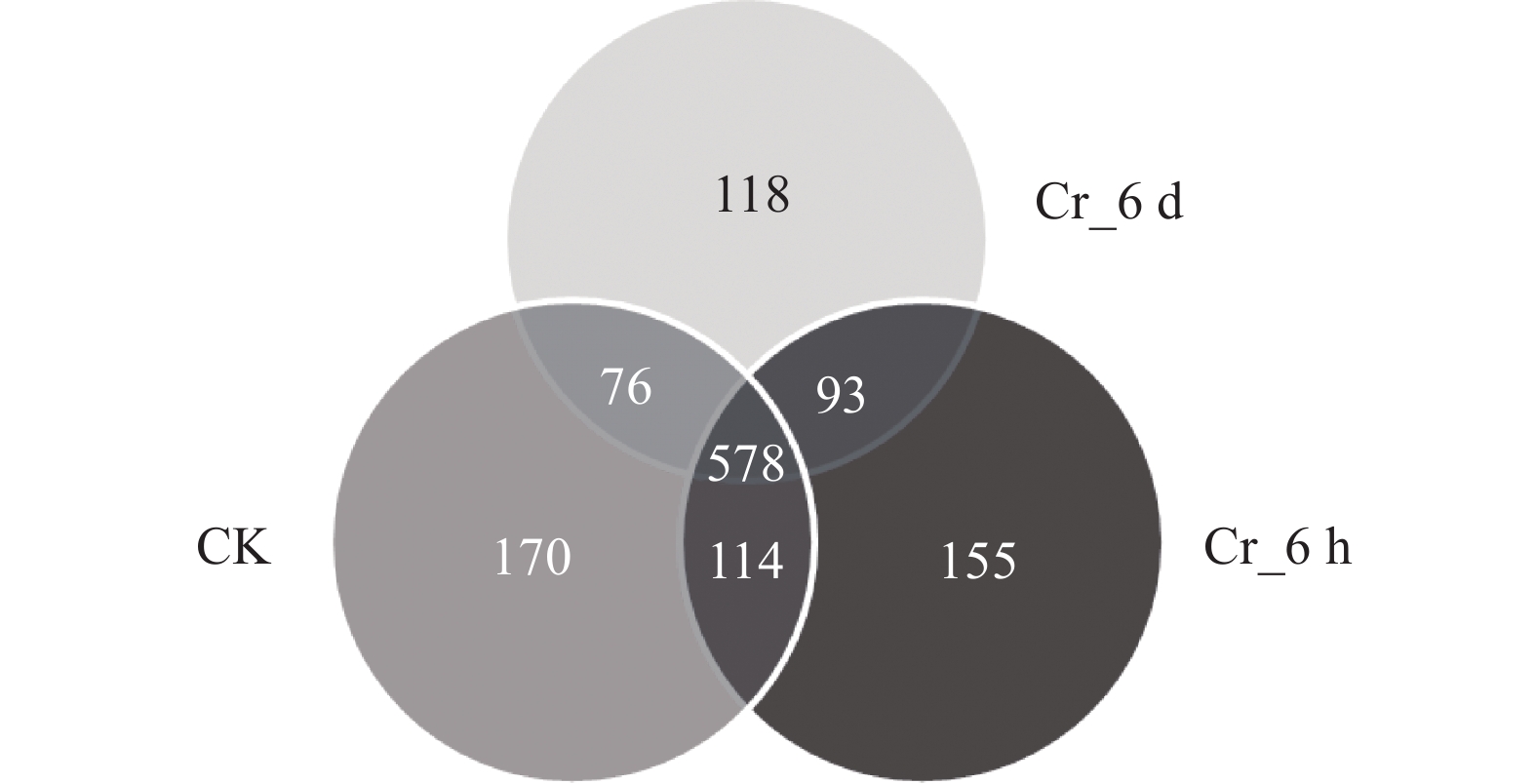
图 1 Cr胁迫时间序列上的真菌群落OTUs Venn图
不同颜色代表不同处理, 重叠部分数字为不同处理共有物种数, 非重叠部分数字为对应处理的特有物种数目。CK: 对照组; Cr_6 h: Cr胁迫后6 h; Cr_6 d: Cr胁迫后6 d。Different colors represent different treatments, the number in the overlapping part represents the number of species in multiple treatments, and the number in the non-overlapping part represents the number of species unique to the corresponding treatment. CK: control treatment; Cr_6 h: Cr stress for 6 hours; Cr_6 d: Cr stress for 6 days.
Figure 1. OTUs Venn diagram of fungal communities in the Cr stress time series
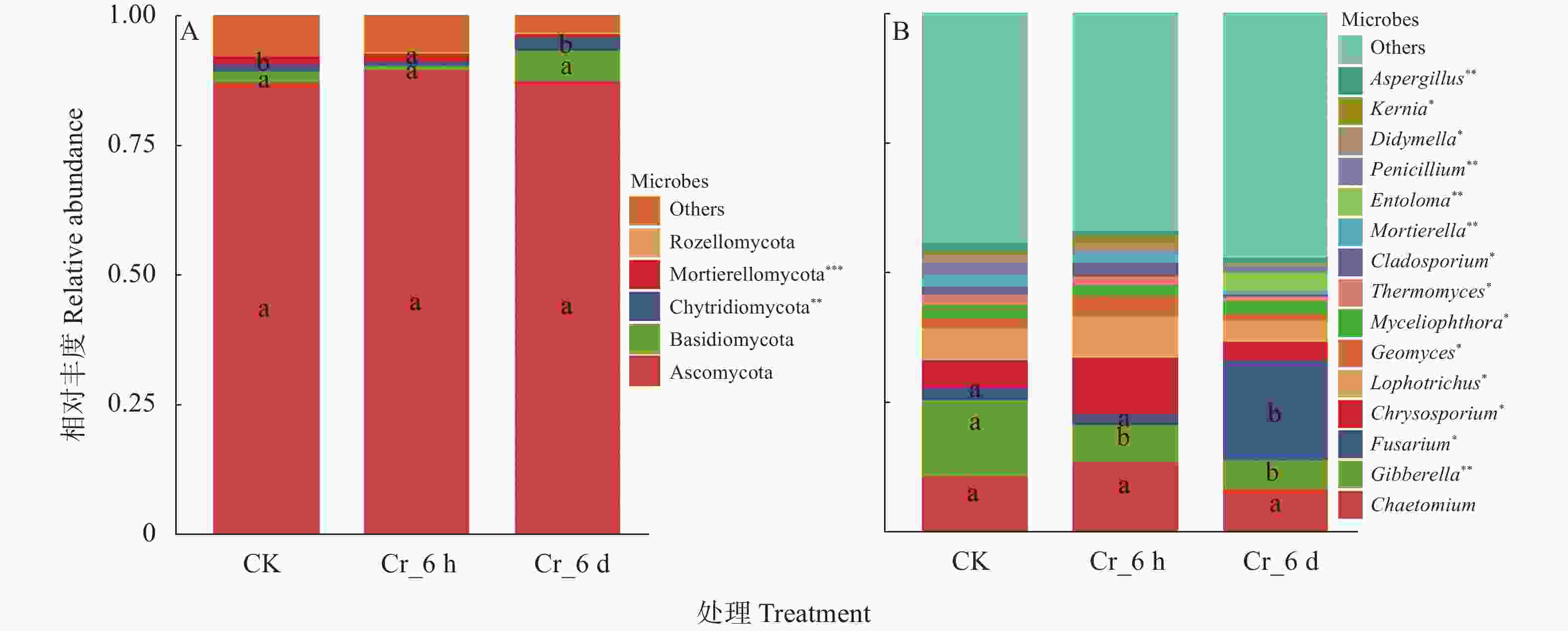
图 2 Cr胁迫时间序列上门水平(A)和属水平(B)真菌群落结构与差异性
CK: 对照组; Cr_6 h: Cr胁迫后6 h; Cr_6 d: Cr胁迫后6 d。不同字母表示同一菌群在不同处理间差异达显著水平(P<0.05)。CK: control treatment; Cr_6 h: Cr stress for 6 hours; Cr_6 d: Cr stress for 6 days. Different letters indicate significant differences in the relative abundance of the same fungi groups among different treatments (P<0.05). *: P<0.05; **: P<0.01; ***: P<0.001.
Figure 2. Phylum level (A) and genus level (B) fungal community structure and differences among treatments in the Cr stress time series
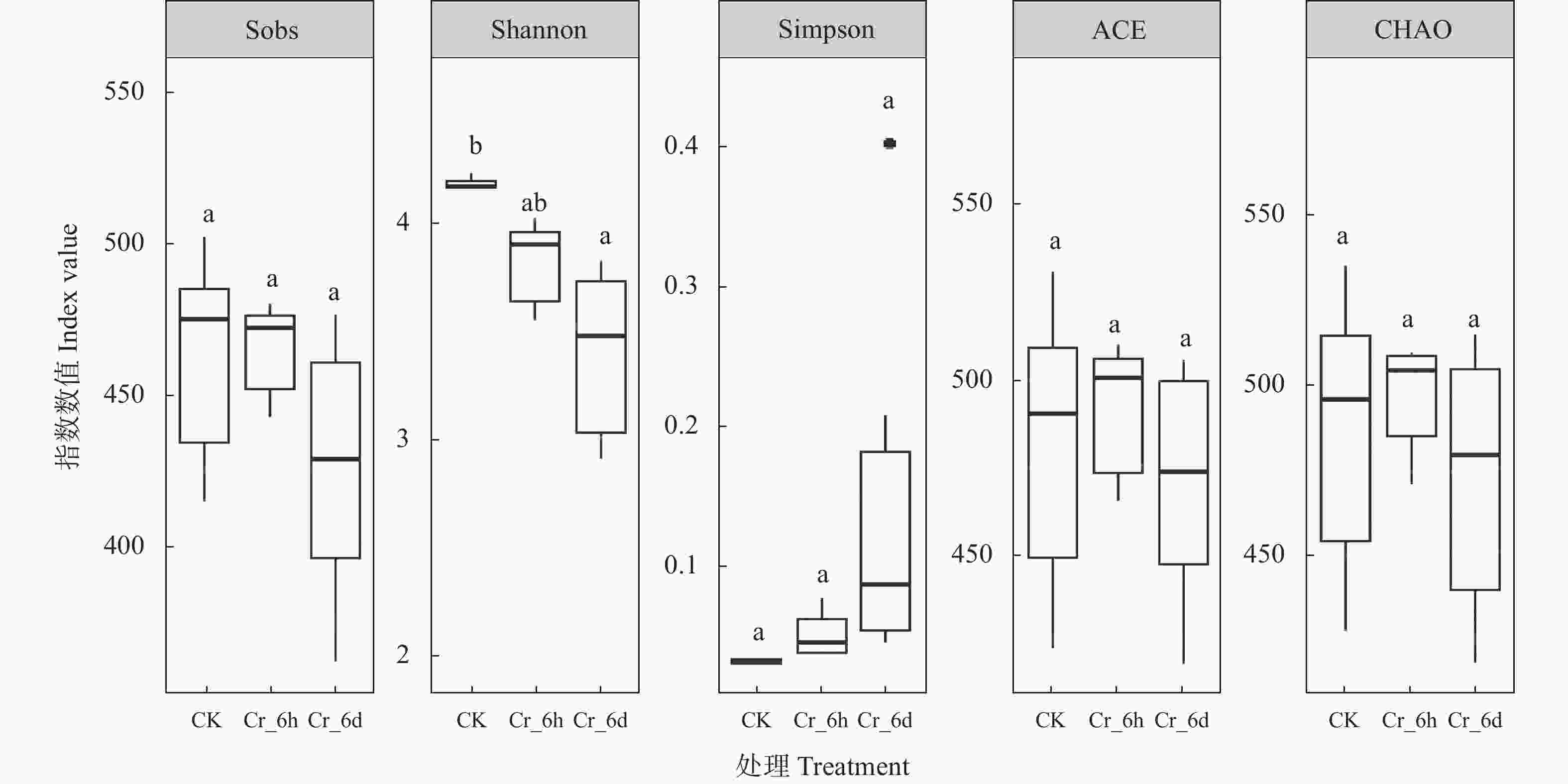
图 3 Cr胁迫时间序列上真菌群落多样性指数分析
CK: 对照组; Cr_6 h: Cr胁迫后6 h; Cr_6 d: Cr胁迫后6 d。不同字母表示不同处理间差异显著(P<0.05)。黑色点表示异常值, 箱式图的上中下3条线分别代表上四分位数、中位数和下四分位数, 竖直的黑线表示误差线。CK: control treatment; Cr_6 h: Cr stress for 6 hours; Cr_6 d: Cr stress for 6 days. Different letters indicate significant differences among treatments at P<0.05 level. The black dots represent outliers, and the upper, middle, and lower lines of the box chart represent the upper quartile, median, and lower quartile, respectively. The vertical black line is the error bar.
Figure 3. Analysis of fungal community diversity indexes in the Cr stress time series
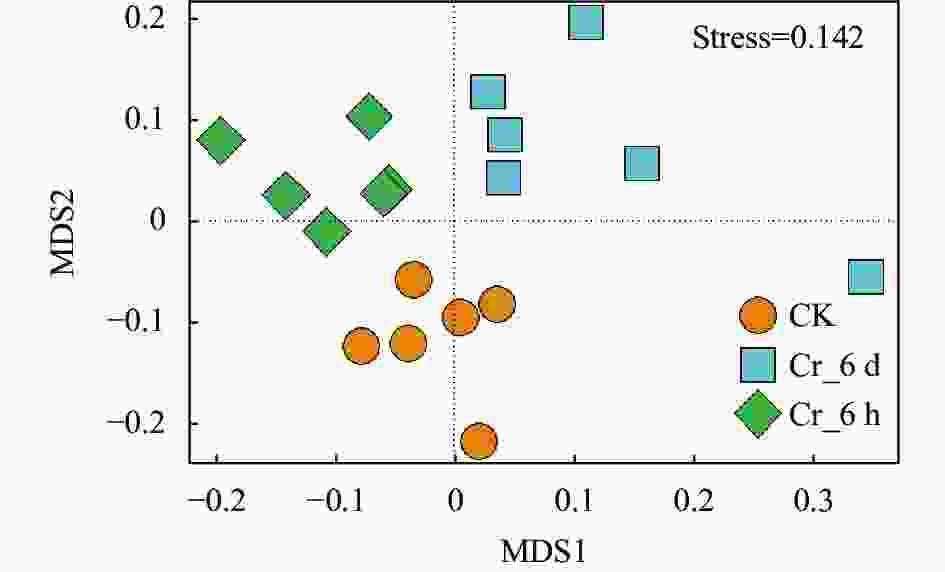
图 4 Cr胁迫时间序列上真菌群落的非度量多维尺度分析
CK: 对照组; Cr_6 h: Cr胁迫后6 h; Cr_6 d: Cr胁迫后6 d。不同颜色或形状的点代表不同处理的样本, 两样本点越接近, 表明两样本物种组成越相似。CK: control treatment; Cr_6 h: Cr stress for 6 hours; Cr_6 d: Cr stress for 6 days. Points of different colors or shapes represent samples of different treatments. The closer the two sample points, the more similar the species composition of the two samples.
Figure 4. Non-metric multidimensional scale analysis of fungal communities on the Cr stress time series
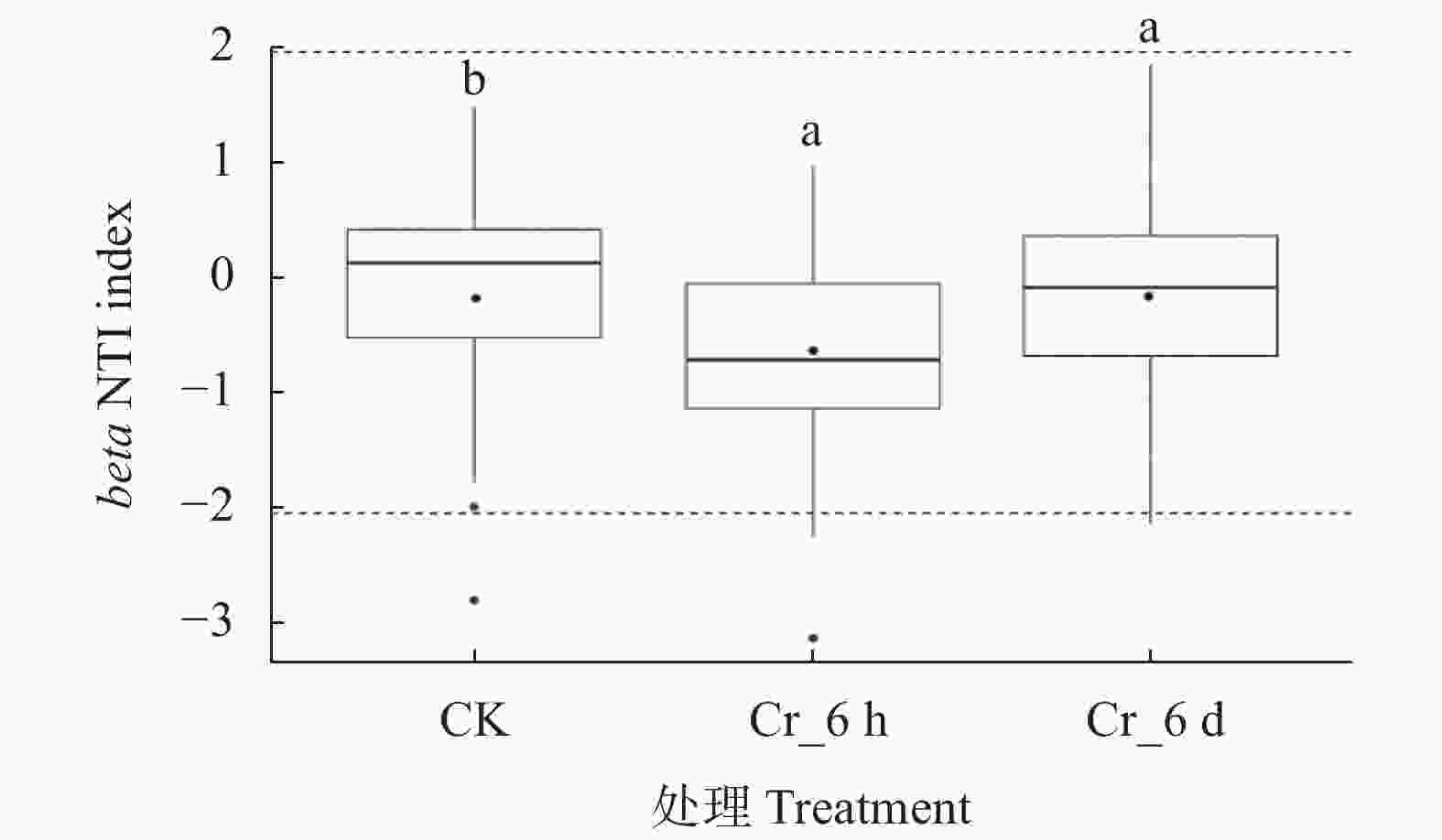
图 5 Cr胁迫时间序列上土壤真菌群落的beta NTI值
CK: 对照组; Cr_6 h: Cr胁迫后6 h; Cr_6 d: Cr胁迫后6 d。箱线图中点表示平均值, 虚线表示beta NTI值为2和−2。不同字母表示不同处理间差异显著(P<0.05)。CK: control treatment; Cr_6 h: Cr stress for 6 hours; Cr_6 d: Cr stress for 6 days. The point in the box plot represents the average value, and the dotted lines represent the beta NTI values of 2 and −2. Different letters indicate significant differences among treatments at P<0.05 level.
Figure 5. beta NTI values of soil fungal communities in the Cr stress time series
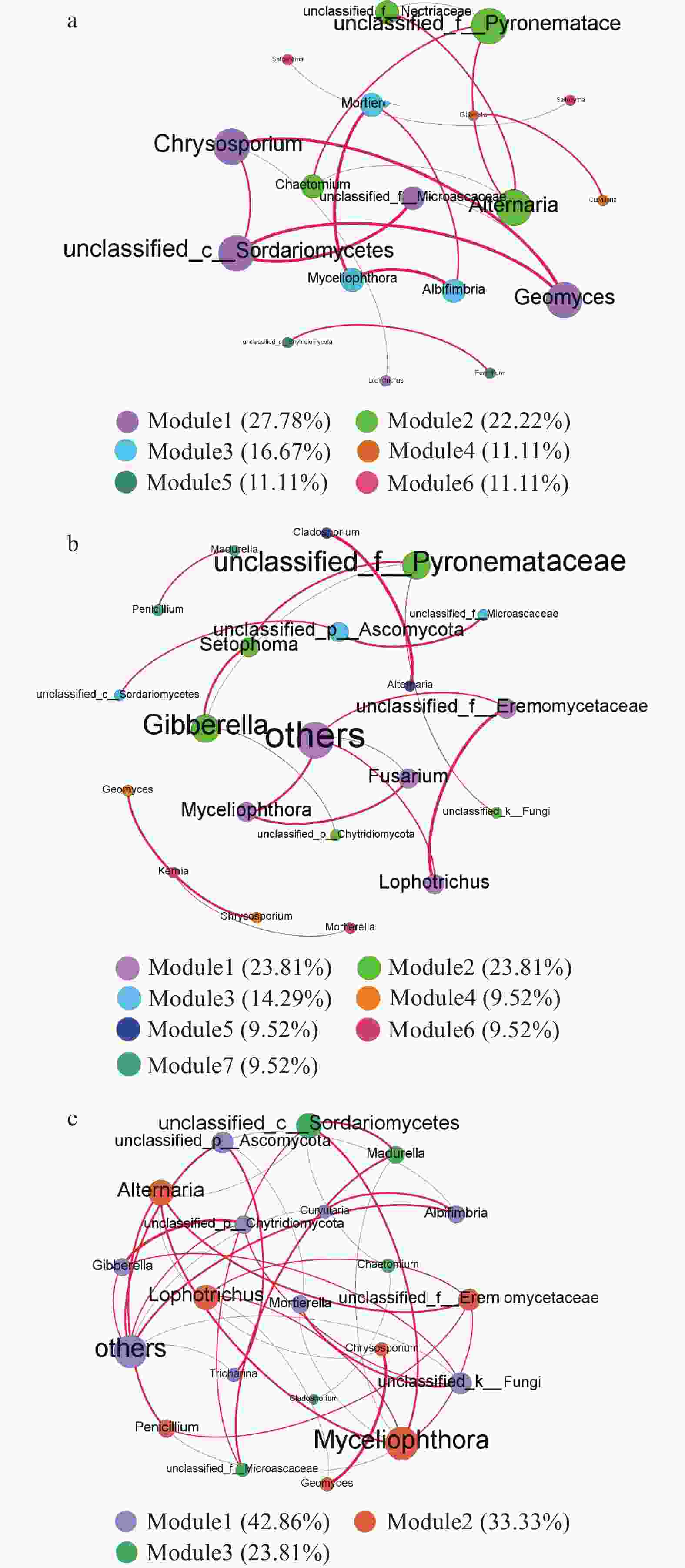
图 6 Cr胁迫时间序列上的真菌种群间共生网络图(a: CK; b: Cr_6 h, Cr胁迫后6 h; c: Cr_6 d, Cr胁迫后6 d)
节点代表不同的真菌属, 节点大小表示度中心性的高低, 节点越大, 在网络中越重要; 红色的边表示正相关, 边代表两节点间相关性显著(P<0.05), 边越粗则相关性越显著; 图中不同颜色节点代表不同模块。Nodes represent different fungal genera. The size of the node indicates the degree of centrality. The larger the node, the more important it is in the network. The red edge indicates the positive correlation, and the edge represents a significant correlation between two nodes. The thicker the edge, the more significant the correlation. Different colored nodes represent different modules.
Figure 6. Inter-population symbiosis network diagram of fungi in the Cr stress time series (a: CK; b: Cr_6 h, Cr stress for 6 hours; c: Cr_6 d, Cr stress for 6 days)
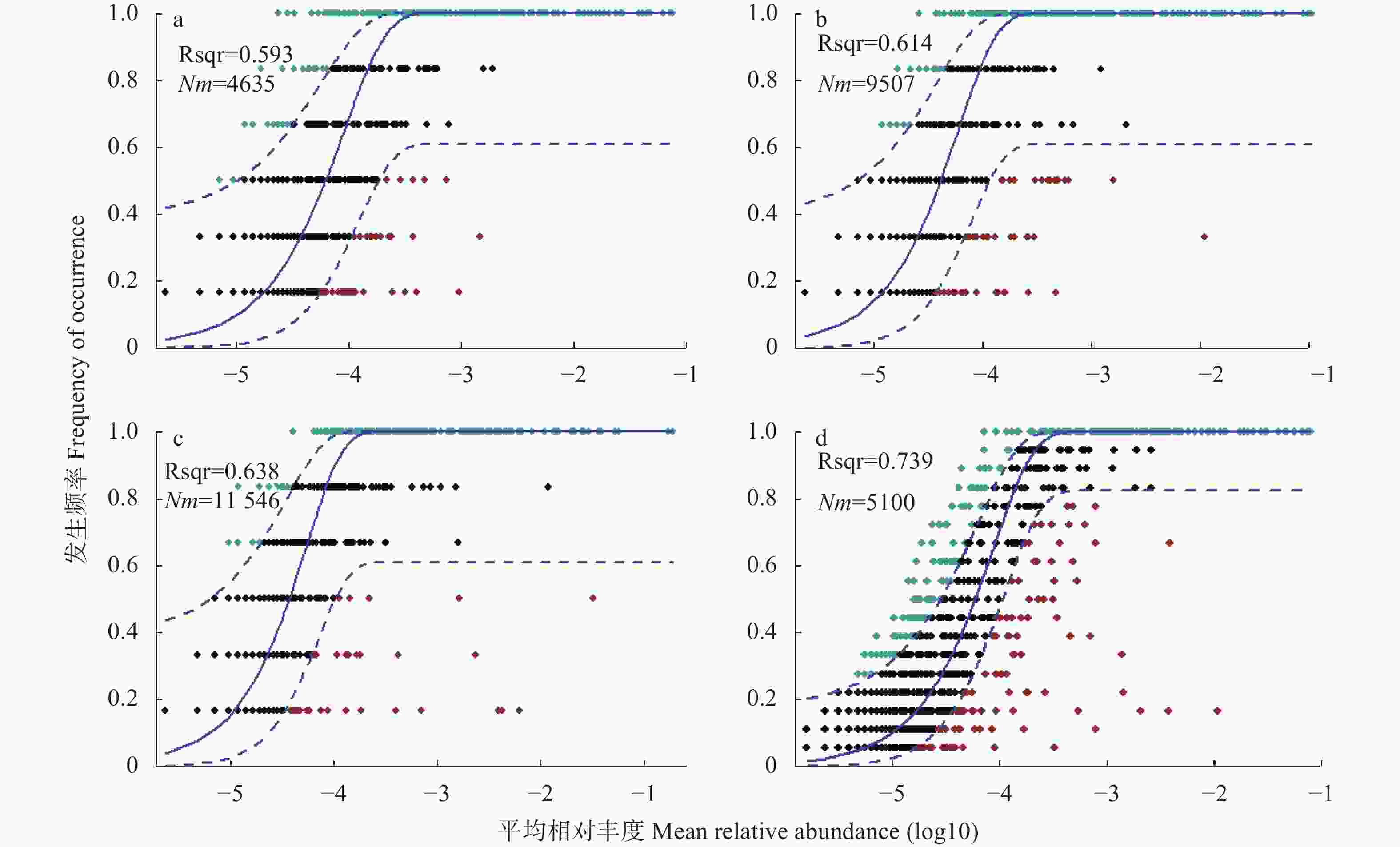
图 7 Cr胁迫时间序列上的中性真菌群落模型(a: CK; b: Cr_6 h, Cr胁迫后6 h; c: Cr_6 d, Cr胁迫后6 d; d: 整体水平)
Rsqr代表了中性模型的拟合优度, 值越高群落构建受随机过程影响越大; Nm表示元群落规模 (N) 与迁移率(m)的乘积, 量化群落间扩散的估计, m值越高则表明物种受到扩散限制越低。图中实线表示模型的最适拟合值, 虚线代表模型的95%置信区间。Rsqr represents the fit goodness of the neutral model. The higher the value, the more the community construction is affected by the stochastic processes. Nm represents the product of the metacommunity size (N) and the migration rate (m) in each sample to quantify the community estimated diffusion, the higher the m value, the lower the species is restricted by diffusion. The solid line in the figure represents the best fit value of the model, and the dashed line represents the 95% confidence interval of the model.
Figure 7. Neutral community model in the Cr stress time series (a: CK; b: Cr_6 h, Cr stress for 6 hours; c: Cr_6 d, chromium stress for 6 days; d: overall level)
表 1 Cr胁迫时间序列上的真菌群落网络属性表
Table 1. Network attributes of fungal communities on the Cr stress time series
网络属性
Network attribute对照
Control (CK)Cr胁迫6 h
Cr stress for 6 hours (Cr_6 h)Cr胁迫6 d
Cr stress for 6 days (Cr_6 d)边数量
Number of edges17 17 36 节点数量
Number of nodes18 21 21 平均度
Average degree1.89 1.62 3.43 聚集系数
Clustering coefficient0.71 0.5 0.47 模块性
Modularity0.75 0.76 0.50 平均路径长度
Average path length1.27 1.41 3.08 表 2 Cr处理时间序列上真菌功能丰度
Table 2. Abundance of fungal functional diversity in the Cr stress time series
功能分组
Guild对照
Control (CK)Cr胁迫6 h
Chromium stress for
6 hours (Cr_6 h)Cr胁迫6 d
Chromium stress for
6 days (Cr_6 d)未定义
Unknown16 645.83±1715.25b 16 478.17±2202.24b 11 226.17±3788.80a 未定义的腐生菌
Undefined saprotroph11 919.33±1237.78a 9633.50±2636.32a 18 537.33±10095.58a 动物病原体-内生菌-植物病原体-未定义腐生菌
Animal pathogen-endophyte-plant pathogen-undefined saprotroph10 675.17±1585.46ab 12 060.50±1946.59b 7946.33±3433.26a 动物病原体-粪便腐生菌-内生菌-附生植物-植物腐生菌-木材腐生菌
Animal pathogen-dung saprotroph-endophyte-epiphyte-plant saprotroph-wood saprotroph7631.33±1345.52a 9334.50±2107.57a 6545.83±4562.01a 植物病原体
Plant pathogen10 021.17±2090.68b 5757.83±1763.85a 4860.67±2198.03a 动物病原体-内生菌-地衣寄生虫-植物病原体-土壤腐生菌-木材腐生菌
Animal pathogen-endophyte-lichen parasite-plant pathogen-soil saprotroph-wood saprotroph1255.17±416.48a 1119.50±217.63a 11 740.17±17952.63a 未定义的腐生菌-木材腐生菌
Undefined saprotroph-wood saprotroph2900.17±634.76a 7091.50±5745.80a 2102.83±1268.02a 土壤腐生菌
Soil saprotroph1034.83±278.94a 2586.50±2147.30a 780.17±446.38a 内生植物病原体
Endophyte-plant pathogen1042.33±221.68b 1001.33±156.52b 601.17±288.41a 动物病原体-内生菌-地衣寄生虫-植物病原体-木材腐生菌
Animal pathogen-endophyte-lichen parasite-plant pathogen-wood saprotroph902.17±183.73a 1205.50±1345.39a 288.67±141.80a 内生菌-凋落物腐生菌-土壤腐生菌-未定义的腐生菌
Endophyte-litter saprotroph-soil saprotroph-undefined saprotroph1051.17±204.80b 962.83±300.70b 365.83±143.43a 动物病原体-植物病原体-未定义腐生菌
Animal pathogen-plant pathogen-undefined saprotroph
外生菌根-真菌寄生虫-土壤腐生菌-未定义腐生菌
Ectomycorrhizal-fungal parasite-soil saprotroph-undefined saprotroph1125.00±296.41b 795.67±326.78ab 420.00±249.72a 505.50±110.97a 992.50±1083.81a 380.67±192.90a 粪便腐生菌-未定义的腐生菌
Dung saprotroph-undefined saprotroph950.00±1082.89a 282.83±142.87a 297.00±218.49a 表中数据为平均值±标准差, 同行数据后不同小写字母表示不同处理差异显著(P<0.05)。Data in the table are mean ± standard deviation. Different small letters in the same row indicate significant differences among different treatments (P<0.05). -
[1] 陈文轩, 李茜, 王珍, 等. 中国农田土壤重金属空间分布特征及污染评价[J]. 环境科学, 2020, 41(6): 2822−2833CHEN W X, LI Q, WANG Z, et al. Spatial distribution characteristics and pollution evaluation of heavy metals in arable land soil of China[J]. Environmental Science, 2020, 41(6): 2822−2833 [2] KADIISKA M B, XIANG Q H, MASON R P. In vivo free radical generation by chromium (Ⅵ): an electron spin resonance spin-trapping investigation[J]. Chemical Research in Toxico-logy, 1994, 7(6): 800−805 doi: 10.1021/tx00042a013 [3] HALL J L. Cellular mechanisms for heavy metal detoxification and tolerance[J]. Journal of Experimental Botany, 2002, 53(366): 1−11 [4] XIA X H, CHEN X, LIU R M, et al. Heavy metals in urban soils with various types of land use in Beijing, China[J]. Journal of Hazardous Materials, 2011, 186(2/3): 2043−2050 [5] 王秀丽, 刘安军, 李琨, 等. 胶原蛋白多肽-铬(Ⅲ)螯合物的降血糖机理探讨[J]. 食品研究与开发, 2006, 27(5): 125−126, 48 doi: 10.3969/j.issn.1005-6521.2006.05.048WANG X L, LIU A J, LI K, et al. Study on the hypoglycemic effects of collagen peptide-chromium(Ⅲ) complex[J]. Food Research and Development, 2006, 27(5): 125−126, 48 doi: 10.3969/j.issn.1005-6521.2006.05.048 [6] CERVANTES C, CAMPOS-GARCı́A J, DEVARS S, et al. Interactions of chromium with microorganisms and plants[J]. FEMS Microbiology Reviews, 2001, 25(3): 335−347 doi: 10.1111/j.1574-6976.2001.tb00581.x [7] 陈建文, 张红, 李君剑, 等. 铜尾矿坝及其周边土壤真菌群落结构与功能多样性[J]. 环境科学, 2021, 42(4): 2056−2065CHEN J W, ZHANG H, LI J J, et al. Soil fungal community structure and functional diversity in a copper tailing dam and its surrounding areas[J]. Environmental Science, 2021, 42(4): 2056−2065 [8] YU Z Q. Microbial remediation of heavy metal (loid) contaminated soil: A review[J]. Agricultural Science & Technology, 2016, 17(1): 85−91 [9] XIE Y, FAN J B, ZHU W X, et al. Effect of heavy metals pollution on soil microbial diversity and bermudagrass genetic variation[J]. Frontiers in Plant Science, 2016, 7: 755 [10] GÓMEZ-SAGASTI M T, ALKORTA I, BECERRIL J M, et al. Microbial monitoring of the recovery of soil quality during heavy metal phytoremediation[J]. Water, Air, & Soil Pollution, 2012, 223(6): 3249−3262 [11] WANG F, YAO J, SI Y, et al. Short-time effect of heavy metals upon microbial community activity[J]. Journal of Hazardous Materials, 2010, 173(1/2/3): 510−516 [12] ORWIN K H, WARDLE D A. New indices for quantifying the resistance and resilience of soil biota to exogenous disturbances[J]. Soil Biology and Biochemistry, 2004, 36(11): 1907−1912 doi: 10.1016/j.soilbio.2004.04.036 [13] 于皓, 安益君, 金德才, 等. 铬污染对土壤细菌群落结构及其构建机制的影响[J]. 环境科学, 2021, 42(3): 1197−1204YU H, AN Y J, JIN D C, et al. Effects of chromium pollution on soil bacterial community structure and assembly processes[J]. Environmental Science, 2021, 42(3): 1197−1204 [14] CHAI L Y, HUANG S H, YANG Z H, et al. Cr(Ⅵ) remediation by indigenous bacteria in soils contaminated by chromium-containing slag[J]. Journal of Hazardous Materials, 2009, 167(1/2/3): 516−522 [15] WANG B C, ZHU S X, LI W J, et al. Effects of chromium stress on the rhizosphere microbial community composition of Cyperus alternifolius[J]. Ecotoxicology and Environmental Safety, 2021, 218: 112253 doi: 10.1016/j.ecoenv.2021.112253 [16] 陈静, 刘荣辉, 陈岩贽, 等. 重金属污染对土壤微生物生态的影响[J]. 生命科学, 2018, 30(6): 667−672CHEN J, LIU R H, CHEN Y Z, et al. Effect of heavy metal pollution on soil microbial ecology[J]. Chinese Bulletin of Life Sciences, 2018, 30(6): 667−672 [17] HU J, MENG D L, LIU X D, et al. Response of soil fungal community to long-term chromium contamination[J]. Transactions of Nonferrous Metals Society of China, 2018, 28(9): 1838−1846 doi: 10.1016/S1003-6326(18)64828-9 [18] 刘晋仙, 柴宝峰, 罗正明. 复合污染尾矿废水中真菌群落多样性及其驱动机制[J]. 生物多样性, 2021, 29(3): 373−384 doi: 10.17520/biods.2020181LIU J X, CHAI B F, LUO Z M. Driving forces and the diversity of fungal communities in complex contaminated tailings drainage[J]. Biodiversity Science, 2021, 29(3): 373−384 doi: 10.17520/biods.2020181 [19] 马景勇, 康凤亭, 田景春. 谷子需水规律与土壤水分对农艺性状影响的研究[J]. 吉林农业大学学报, 1987, 9(2): 38−41MA J Y, KANG F T, TIAN J C. Study on millet’ s water-requiring rules and the effect of soil moisture on millet’ s agronomic characters[J]. Journal of Jilin Agricultural University, 1987, 9(2): 38−41 [20] 肖文丹, 叶雪珠, 孙彩霞, 等. 铬耐性菌对土壤中六价铬的还原作用[J]. 中国环境科学, 2017, 37(3): 1120−1129XIAO W D, YE X Z, SUN C X, et al. The effect of chromium-resistant bacteria on reduction of hexavalent chromium in soils[J]. China Environmental Science, 2017, 37(3): 1120−1129 [21] STEGEN J C, LIN X J, KONOPKA A E, et al. Stochastic and deterministic assembly processes in subsurface microbial communities[J]. The ISME Journal, 2012, 6(9): 1653−1664 doi: 10.1038/ismej.2012.22 [22] STEGEN J C, LIN X J, FREDRICKSON J K, et al. Quantifying community assembly processes and identifying features that impose them[J]. The ISME Journal, 2013, 7(11): 2069−2079 doi: 10.1038/ismej.2013.93 [23] STEGEN J C, LIN X J, FREDRICKSON J K, et al. Estimating and mapping ecological processes influencing microbial community assembly[J]. Frontiers in Microbiology, 2015, 6: 370 [24] SLOAN W T, LUNN M, WOODCOCK S, et al. Quantifying the roles of immigration and chance in shaping prokaryote community structure[J]. Environmental Microbiology, 2006, 8(4): 732−740 doi: 10.1111/j.1462-2920.2005.00956.x [25] TIGINI V, PRIGIONE V, VARESE G C. Mycological and ecotoxicological characterisation of landfill leachate before and after traditional treatments[J]. Science of the Total Environment, 2014, 487: 335−341 doi: 10.1016/j.scitotenv.2014.04.026 [26] TRIVEDI B D, PATEL K C. Biosorption of hexavalent chromium from aqueous solution by a tropical basidiomycete BDT-14 (DSM 15396)[J]. World Journal of Microbiology and Biotechnology, 2007, 23(5): 683−689 doi: 10.1007/s11274-006-9284-4 [27] BALDRIAN P. Interactions of heavy metals with white-rot fungi[J]. Enzyme and Microbial Technology, 2003, 32(1): 78−91 doi: 10.1016/S0141-0229(02)00245-4 [28] GOSTINČAR C, GRUBE M, DE HOOG S, et al. Extremotolerance in fungi: evolution on the edge[J]. FEMS Microbiology Ecology, 2010, 71(1): 2−11 doi: 10.1111/j.1574-6941.2009.00794.x [29] FUKUDA T, ISHINO Y, OGAWA A, et al. Cr(VI) reduction from contaminated soils by Aspergillus sp. N2 and Penicillium sp. N3 isolated from chromium deposits[J]. The Journal of General and Applied Microbiology, 2008, 54(5): 295−303 doi: 10.2323/jgam.54.295 [30] ZAFAR S, AQIL F, AHMAD I. Metal tolerance and biosorption potential of filamentous fungi isolated from metal contaminated agricultural soil[J]. Bioresource Technology, 2007, 98(13): 2557−2561 doi: 10.1016/j.biortech.2006.09.051 [31] TEDERSOO L, BAHRAM M, PÕLME S, et al. Response to comment on “global diversity and geography of soil fungi”[J]. Science, 2015, 349(6251): 936 [32] 金慧清, 程昌合, 徐清泉, 等. 烟草内生真菌对烟草生长和烟叶重金属含量的影响[J]. 菌物学报, 2017, 36(2): 186−192JIN H Q, CHENG C H, XU Q Q, et al. Effects of endophytic fungi on tobacco growth and heavy metal content in leaves[J]. Mycosystema, 2017, 36(2): 186−192 [33] KUMAR R, KUNDU A, DUTTA A, et al. Chemo-profiling of bioactive metabolites from Chaetomium globosum for biocontrol of Sclerotinia rot and plant growth promotion[J]. Fungal Biology, 2021, 125(3): 167−176 doi: 10.1016/j.funbio.2020.07.009 [34] PAISSÉ S, COULON F, GOÑI-URRIZA M, et al. Structure of bacterial communities along a hydrocarbon contamination gradient in a coastal sediment[J]. FEMS Microbiology Ecology, 2008, 66(2): 295−305 doi: 10.1111/j.1574-6941.2008.00589.x [35] KNELMAN J E, NEMERGUT D R. Changes in community assembly may shift the relationship between biodiversity and ecosystem function[J]. Frontiers in Microbiology, 2014, 5: 424 [36] CHEN W D, REN K X, ISABWE A, et al. Stochastic processes shape microeukaryotic community assembly in a subtropical river across wet and dry seasons[J]. Microbiome, 2019, 7(1): 138 doi: 10.1186/s40168-019-0749-8 [37] TILMAN D. Biodiversity: population versus ecosystem stability[J]. Ecology, 1996, 77(2): 350−363 [38] 李晶, 刘玉荣, 贺纪正, 等. 土壤微生物对环境胁迫的响应机制[J]. 环境科学学报, 2013, 33(4): 959−967LI J, LIU Y R, HE J Z, et al. Insights into the responses of soil microbial community to the environmental disturbances[J]. Acta Scientiae Circumstantiae, 2013, 33(4): 959−967 [39] SULTANA M Y, AKRATOS C S, PAVLOU S, et al. Chromium removal in constructed wetlands: A review[J]. International Biodeterioration & Biodegradation, 2014, 96: 181−190 [40] AMATUSSALAM A, ABUBACKER M N, RAJENDRAN R B. In situ Carica papaya stem matrix and Fusarium oxysporum (NCBT-156) mediated bioremediation of chromium[J]. Indian Journal of Experimental Biology, 2011, 49(12): 925−931 [41] POLTI M A, APARICIO J D, BENIMELI C S, et al. Simultaneous bioremediation of Cr(VI) and lindane in soil by actinobacteria[J]. International Biodeterioration & Biodegradation, 2014, 88: 48−55 [42] DE VRIES F T, GRIFFITHS R I, BAILEY M, et al. Soil bacterial networks are less stable under drought than fungal networks[J]. Nature Communications, 2018, 9: 3033 doi: 10.1038/s41467-018-05516-7 [43] SCHIMEL J P, SCHAEFFER S M. Microbial control over carbon cycling in soil[J]. Frontiers in Microbiology, 2012, 3: 348 [44] MA L, ZHANG J B, LI Z Q, et al. Long-term phosphorus deficiency decreased bacterial-fungal network complexity and efficiency across three soil types in China as revealed by network analysis[J]. Applied Soil Ecology, 2020, 148: 103506 doi: 10.1016/j.apsoil.2020.103506 [45] SHENG M, CHEN X D, ZHANG X L, et al. Changes in arbuscular mycorrhizal fungal attributes along a chronosequence of black locust (Robinia pseudoacacia) plantations can be attributed to the plantation-induced variation in soil properties[J]. Science of the Total Environment, 2017, 599/600: 273−283 doi: 10.1016/j.scitotenv.2017.04.199 [46] XUN W B, LI W, XIONG W, et al. Diversity-triggered deterministic bacterial assembly constrains community functions[J]. Nature Communications, 2019, 10: 3833 doi: 10.1038/s41467-019-11787-5 [47] ALLEN C R, GARMESTANI A S, HAVLICEK T D, et al. Patterns in body mass distributions: sifting among alternative hypotheses[J]. Ecology Letters, 2006, 9(5): 630−643 doi: 10.1111/j.1461-0248.2006.00902.x [48] CHEN W M, JIAO S, LI Q P, et al. Dispersal limitation relative to environmental filtering governs the vertical small-scale assembly of soil microbiomes during restoration[J]. Journal of Applied Ecology, 2020, 57(2): 402−412 doi: 10.1111/1365-2664.13533 [49] LUAN L, JIANG Y J, CHENG M H, et al. Organism body size structures the soil microbial and nematode community assembly at a continental and global scale[J]. Nature Communications, 2020, 11: 6406 doi: 10.1038/s41467-020-20271-4 [50] 孙倩, 吴宏亮, 陈阜, 等. 宁夏中部干旱带不同作物根际土壤真菌群落多样性及群落结构[J]. 微生物学通报, 2019, 46(11): 2963−2972SUN Q, WU H L, CHEN F, et al. Fungal community diversity and structure in rhizosphere soil of different crops in the arid zone of central ningxia[J]. Microbiology China, 2019, 46(11): 2963−2972 [51] ANTHONY M A, FREY S D, STINSON K A. Fungal community homogenization, shift in dominant trophic guild, and appearance of novel taxa with biotic invasion[J]. Ecosphere, 2017, 8(9): e01951 -





 下载:
下载: