Promoting effect of potato intercropping on functional diversity of soil microbial metabolism and nitrogen regulation
-
摘要: 间作是高效利用时间和空间资源的作物种植模式, 是最典型的农业多样性种植模式之一。然而, 地上作物多样性如何影响地下微生物多样性及其氮调控作用尚不清楚。因此, 本研究采用Biolog-Eco微平板法, 在多年田间小区定位试验的第7年, 分析4个氮水平(N0: 0 kg∙hm−2; N1: 62.5 kg∙hm−2; N2: 125 kg∙hm−2; N3: 187.5 kg∙hm−2)下, 马铃薯单作和玉米||马铃薯间作土壤中微生物的功能多样性。结果表明, 与N0相比, 施氮(N1, N2和N3)提高了微生物群落的AWCD值、Simpson指数和Shannon指数, 其中N1处理最高。与相同施氮量的马铃薯单作相比, 玉米马铃薯间作土壤微生物的AWCD值、Simpson指数和Shannon指数更高, 但仅在N0时存在显著差异(P<0.05)。另外, 施氮显著影响了6类碳源的代谢活性(P<0.05)。在马铃薯单作种植土壤中, 施氮提高了微生物对除碳水化合物外所有碳源的代谢能力; 玉米马铃薯间作土壤中, 施氮提高了微生物对聚合物、酚类和胺类化合物(惰性碳源)的代谢能力, 但降低了对碳水化合物、羧酸和氨基酸(活性碳源)的代谢能力。Mantel分析表明, 土壤温度、含水量、土壤有机碳、铵态氮含量和马铃薯生物量是影响微生物碳源代谢活性和功能多样性的主要影响因子, 但铵态氮仅在玉米马铃薯间作土壤中有显著影响(P<0.05)。网络分析表明, 马铃薯单作(0.930)土壤微生物碳源代谢活性的平均聚类系数略高于间作(0.907), 表明马铃薯与玉米间作削弱了土壤微生物群落代谢过程的稳定性。结果表明地上作物多样性种植提高了地下微生物的代谢功能多样性, 且这种互作关系受施氮的显著调控。Abstract: Intercropping is an efficient model for utilizing time and space resources and is a typical diversified agriculture cropping pattern. However, how the diversified planting of aboveground crops affects subsurface microbial diversity and N regulation remains uncertain. Therefore, in this study, the Biolog-EcoMicroPlate cultivation method was used to analyze soil microbial metabolic activity, diversity, and utilization of six carbon sources in potato mono- (MP) and inter-cropping (IP) soils under four N levels (N0, 0 kg∙hm−2; N1, 62.5 kg∙hm−2; N2, 125 kg∙hm−2; and N3, 187.5 kg∙hm−2). The results showed that compared with N0 treatment, N application (N1, N2, and N3 treatments) increased the average well color development (AWCD) values by 32.1%–100.2%, Shannon index by 3.3%–7.1%, and Simpson index by 14.8%–19.2%, and the increase peaked at the N1 treatment. Compared to potato monocropping under the same N application rates, potato intercropping with maize increased AWCD values and Shannon and Simpson indices, but there was a statistically significant difference only in the N0 treatment (P<0.05). Moreover, N application significantly affected the microbial metabolic activity of the six carbon sources (P<0.05). N application increased the microbial utilization of the six carbon sources, except for carbohydrates in monocropping soil. N application increased the microbial utilization of polymers, amines, and phenolic compounds (recalcitrant carbon sources), but decreased the utilization of carbohydrates, carboxylic acids, and amino acids (active carbon sources) in intercropping soil. The Mantel test showed that soil temperature, soil water content, soil organic carbon, soil ammonium N, and potato biomass were the main factors affecting the microbial AWCD values and diversity indices; however, ammonium N only had a significant effect on potato intercropping soil (P<0.05). Furthermore, the average clustering coefficient value in monocultured soil (0.930) was slightly higher than that in intercropped soil (0.907), indicating that potato intercropping with maize weakened the stability of soil microbial metabolic processes compared with potato monocropping ecosystem, leading to microbial carbon metabolism being more sensitive to changes in the soil microenvironment and further weakening the promoting effect of intercropping. Overall, diversified planting of aboveground crops increased subsurface microbial metabolic activity and diversity; however, this process was significantly regulated by N application. This indicates that a reasonable aboveground diversity could accelerate the soil carbon cycle and realize the efficient utilization of soil nutrients and sustainable agricultural development.
-
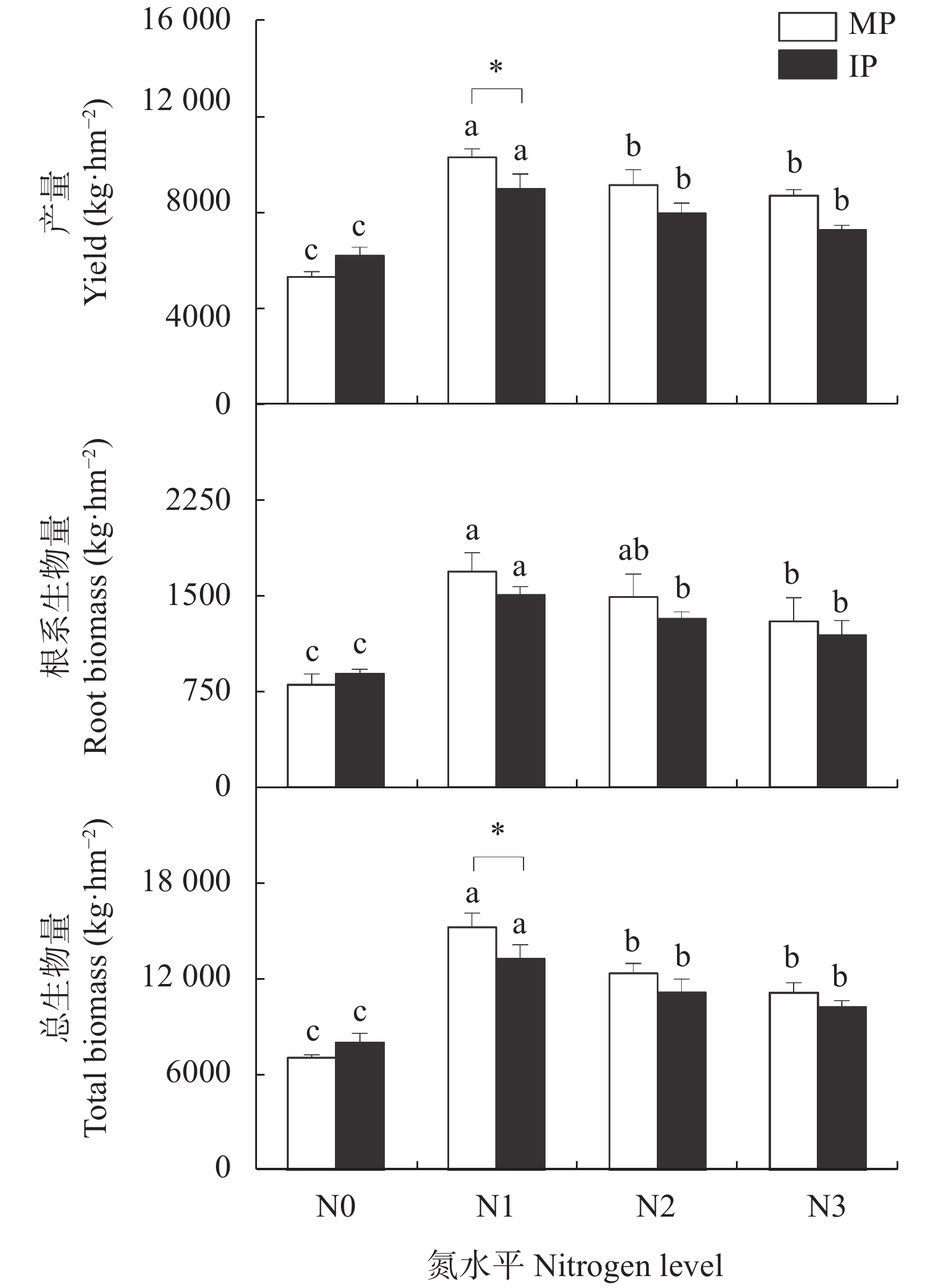
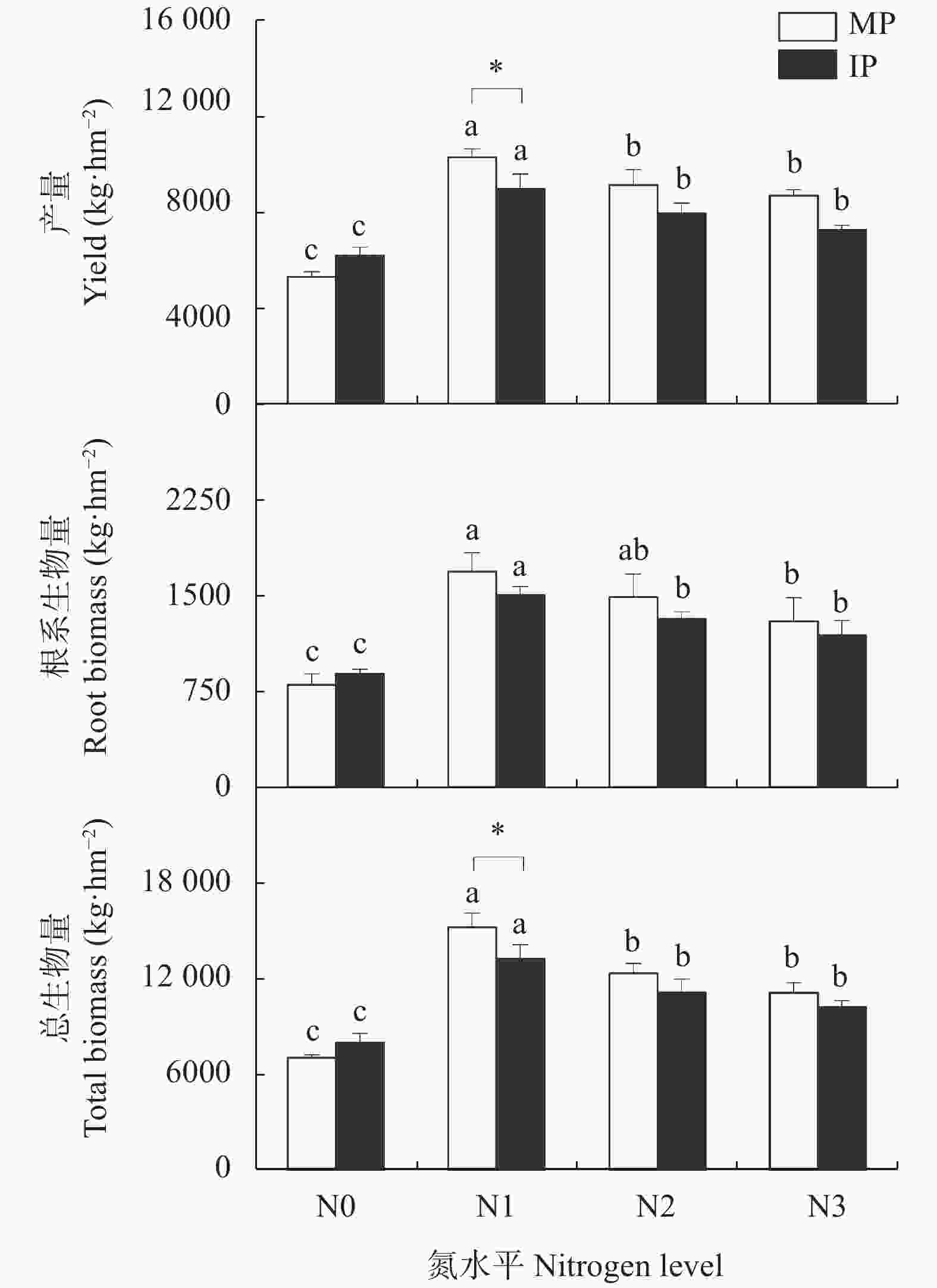
图 1 不同施氮水平下单作(MP)和间作(IP)马铃薯的产量、根系生物量和总生物量
不同小写字母表示同一种植模式下不同氮水平间差异显著(P<0.05), *表示相同氮水平下两种种植模式差异显著(P<0.05)。N0、N1、N2和N3分别表示马铃薯种植土壤中施氮量为0 kg∙hm−2、62.5 kg∙hm−2、125 kg∙hm−2和187.5 kg∙hm−2.
Figure 1. Yield, root biomass and total biomass of mono- (MP) and inter-cropped (IP) potato under different nitrogen application levels
Different lowercase letters indicate significant differences among different nitrogen levels for the same cropping pattern at P<0.05. * means significant difference between two cropping patterns under the same nitrogen level at P<0.05 level. N application rates of N0, N1, N2, and N3 are 0, 62.5, 125 and 187.5 kg∙hm−2 in potato planted soil, respectively.
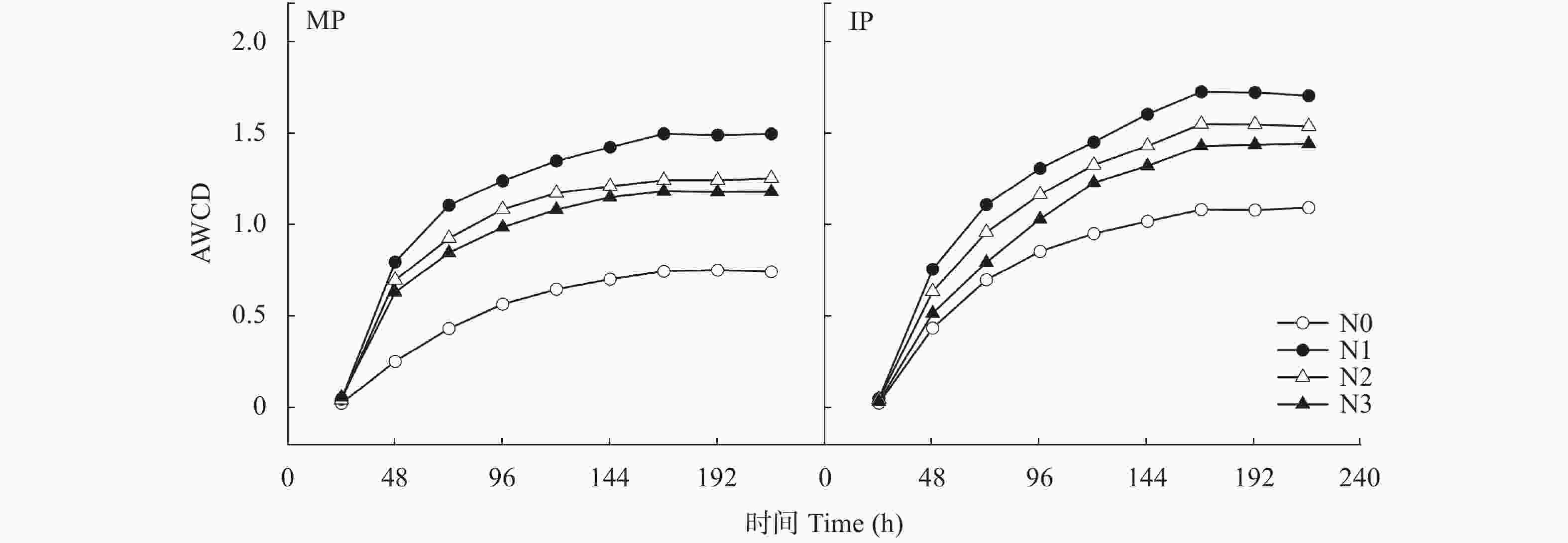
图 2 不同氮水平下单作(MP)和间作(IP)马铃薯种植土壤微生物代谢活性(AWCD值)的动态变化
N0、N1、N2和N3分别表示马铃薯种植土壤的施氮量为0 kg∙hm−2、62.5 kg∙hm−2、125 kg∙hm−2和187.5 kg∙hm−2.
Figure 2. Dynamic changes of metabolic activity (AWCD values) in mono- (MP) and inter-cropped (IP) potato soils under different nitrogen application levels
N application rates of N0, N1, N2, and N3 are 0, 62.5, 125 and 187.5 kg∙hm−2 in potato planted soil, respectively.
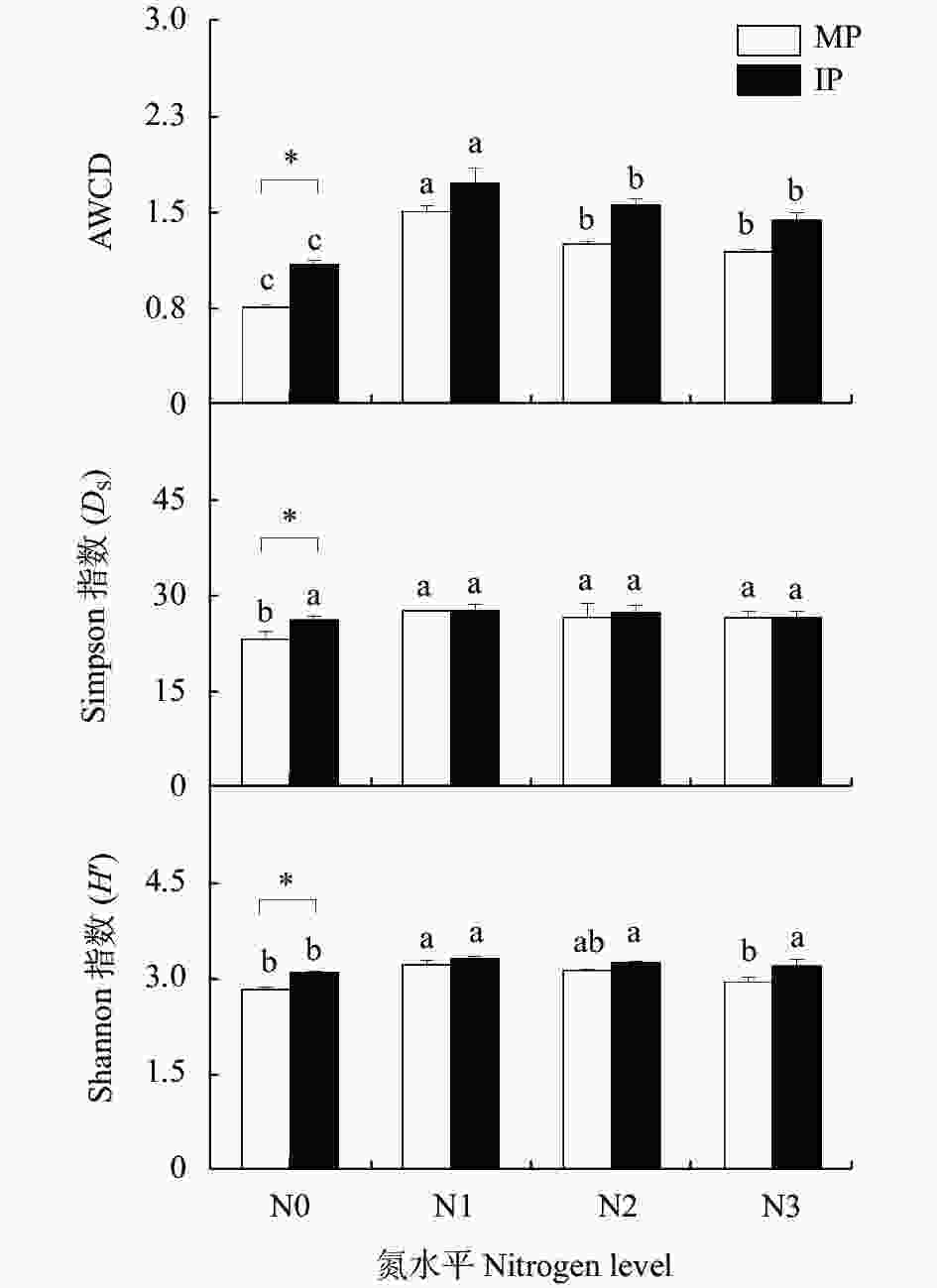
图 3 不同氮水平下单作(MP)和间作(IP)马铃薯土壤的AWCD值和微生物多功能性指数
不同小写字母表示同一种植模式下不同氮水平间在P<0.05水平差异显著; *表示相同氮水平的不同种植模式在P<0.05水平差异显著。N0、N1、N2和N3分别表示马铃薯种植土壤的施氮量为0 kg∙hm−2、62.5 kg∙hm−2、125 kg∙hm−2和187.5 kg∙hm−2. Different lowercase letters mean significant differences among different nitrogen levels at the same cropping pattern at P<0.05 level. * means significant difference between two cropping patterns at P<0.05 level under the same nitrogen level. N application rates of N0, N1, N2, and N3 are 0, 62.5, 125 and 187.5 kg∙hm−2 in potato planted soil, respectively.
Figure 3. AWCD values and indexes of soil microbial functional diversity in mono- (MP) and inter-cropped (IP) potato soils under different nitrogen application levels
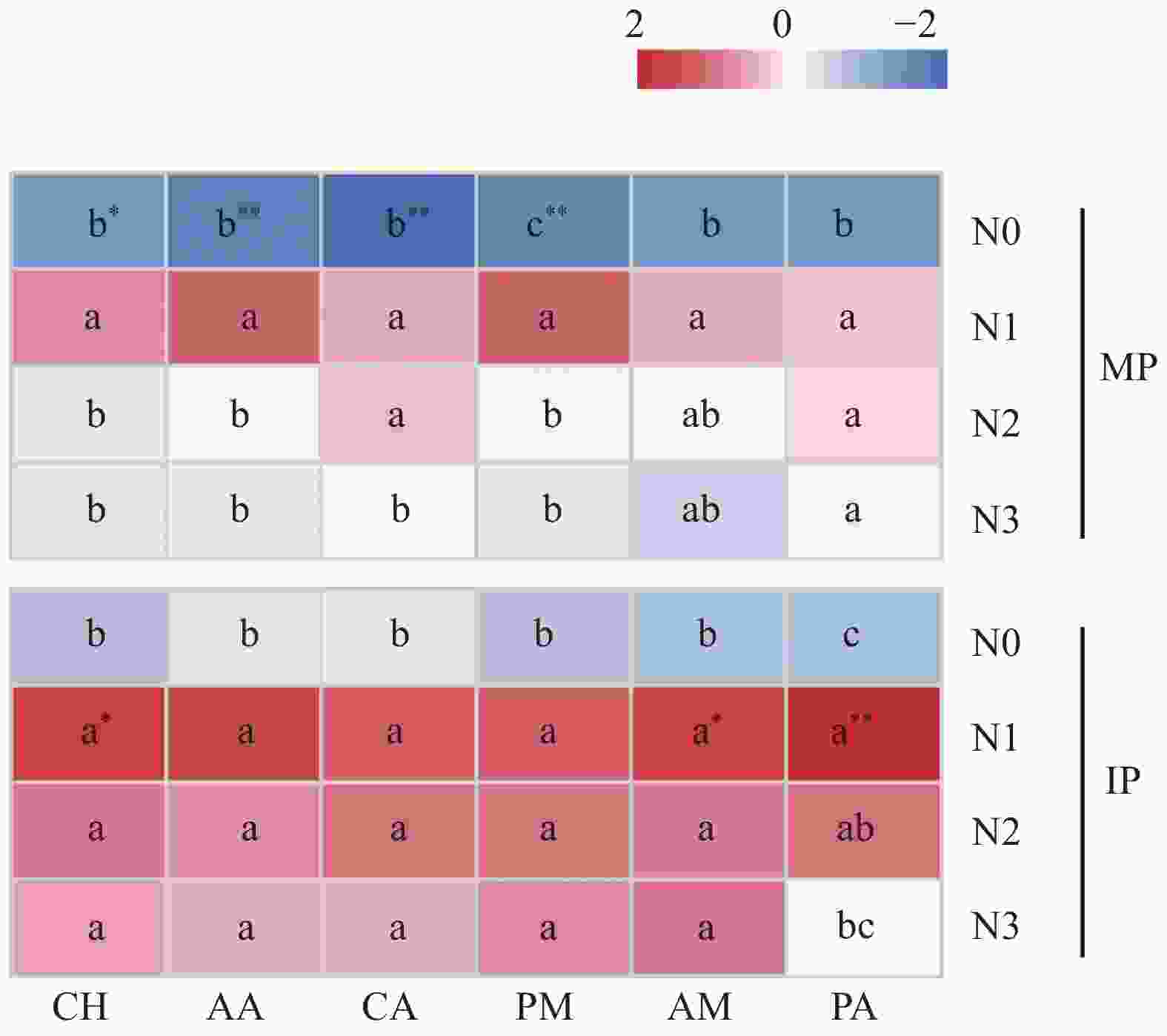
图 4 不同氮水平下单作(MP)和间作(IP)马铃薯土壤中6类碳源AWCD值变化的热图
CH: 碳水化合物; AA: 氨基酸; CA: 羧酸; PM: 聚合物; AM: 胺类; PA: 酚类化合物。不同小写字母表示同一种植模式下不同氮水平间在P<0.05水平差异显著。*和**表示同一氮水平下不同种植模式之间分别在P<0.05和P<0.01水平差异显著。N0、N1、N2和N3分别表示马铃薯种植土壤施氮量为0 kg∙hm−2、62.5 kg∙hm−2、125 kg∙hm−2和187.5 kg∙hm−2.
Figure 4. Heat map of AWCD values of varied carbon sources in mono- (MP) and inter-cropped (IP) potato soils under different nitrogen application levels
CH: carbohydrates; AA: amino acids; CA: carboxylic acids; PM: polymers; AM: amines; PA: phenolic compounds. The lowercase letters (a, b, c) indicate significant differences among different nitrogen levels under the same cropping patterns at P<0.05 level. * and ** indicate significant differences between two cropping patterns under the same nitrogen level at P<0.05 and P<0.01 levels, respectively. N application rates of N0, N1, N2, and N3 are 0, 62.5, 125 and 187.5 kg∙hm−2 in potato planted soil, respectively.
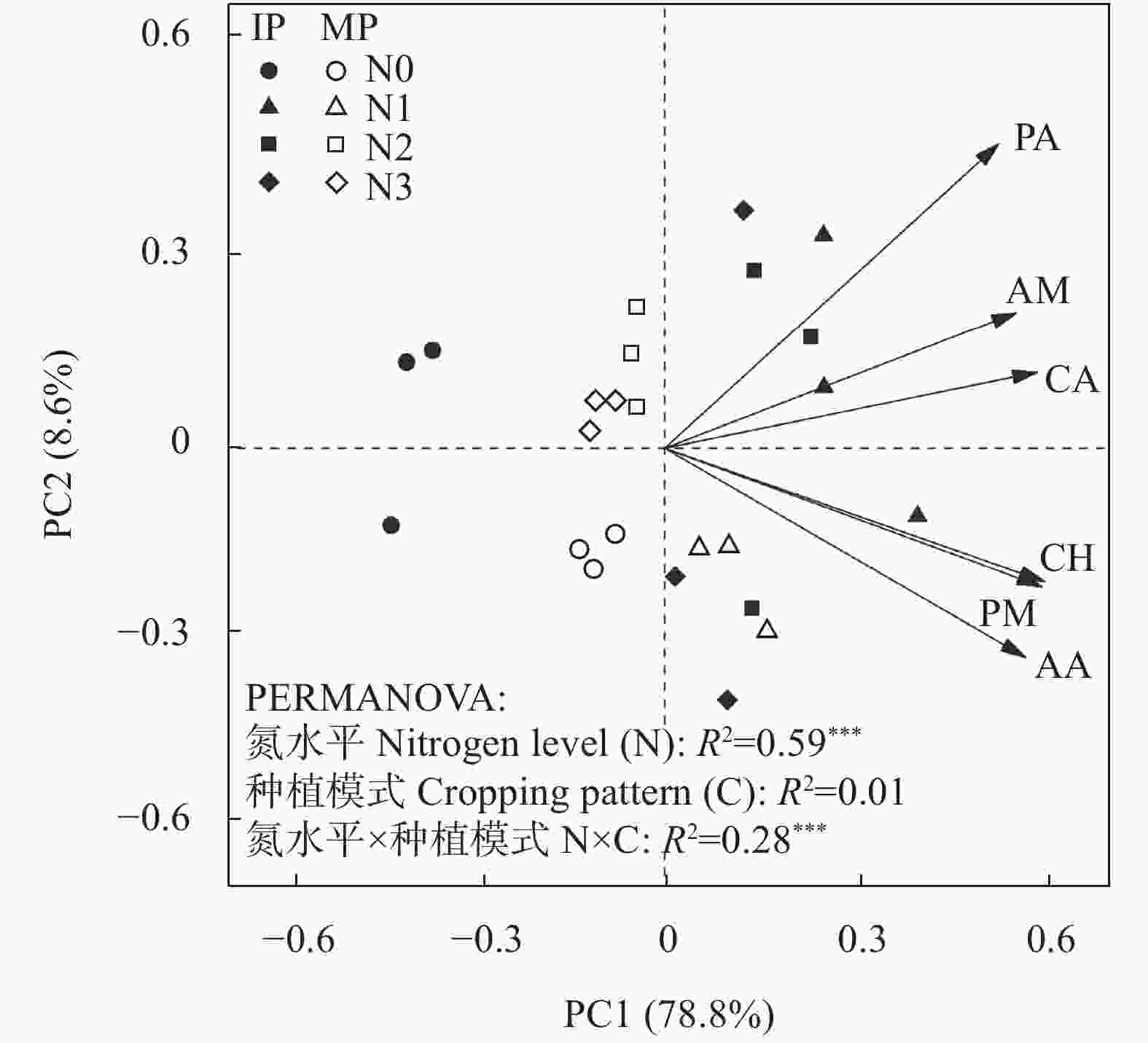
图 5 不同氮水平下单作(MP)和间作(IP)马铃薯土壤中不同碳源的主成分分析
CH: 碳水化合物; AA: 氨基酸; CA: 羧酸; PM: 聚合物; AM: 胺类; PA: 酚类化合物。***表示极显著影响(P<0.001)。N0、N1、N2和N3分别表示马铃薯种植土壤施氮量为0 kg∙hm−2、62.5 kg∙hm−2、125 kg∙hm−2和187.5 kg∙hm−2。CH: carbohydrates; AA: amino acids; CA: carboxylic acids; PM: polymers; AM: amines; PA: phenolic compounds. *** indicate significantly influence at P<0.001 level. N application rates of N0, N1, N2, and N3 are 0, 62.5, 125 and 187.5 kg∙hm−2 in potato planted soil, respectively.
Figure 5. Principal component analysis (PCA) of varied carbon sources in mono- (MP) and inter-cropped (IP) potato soils under different nitrogen application levels
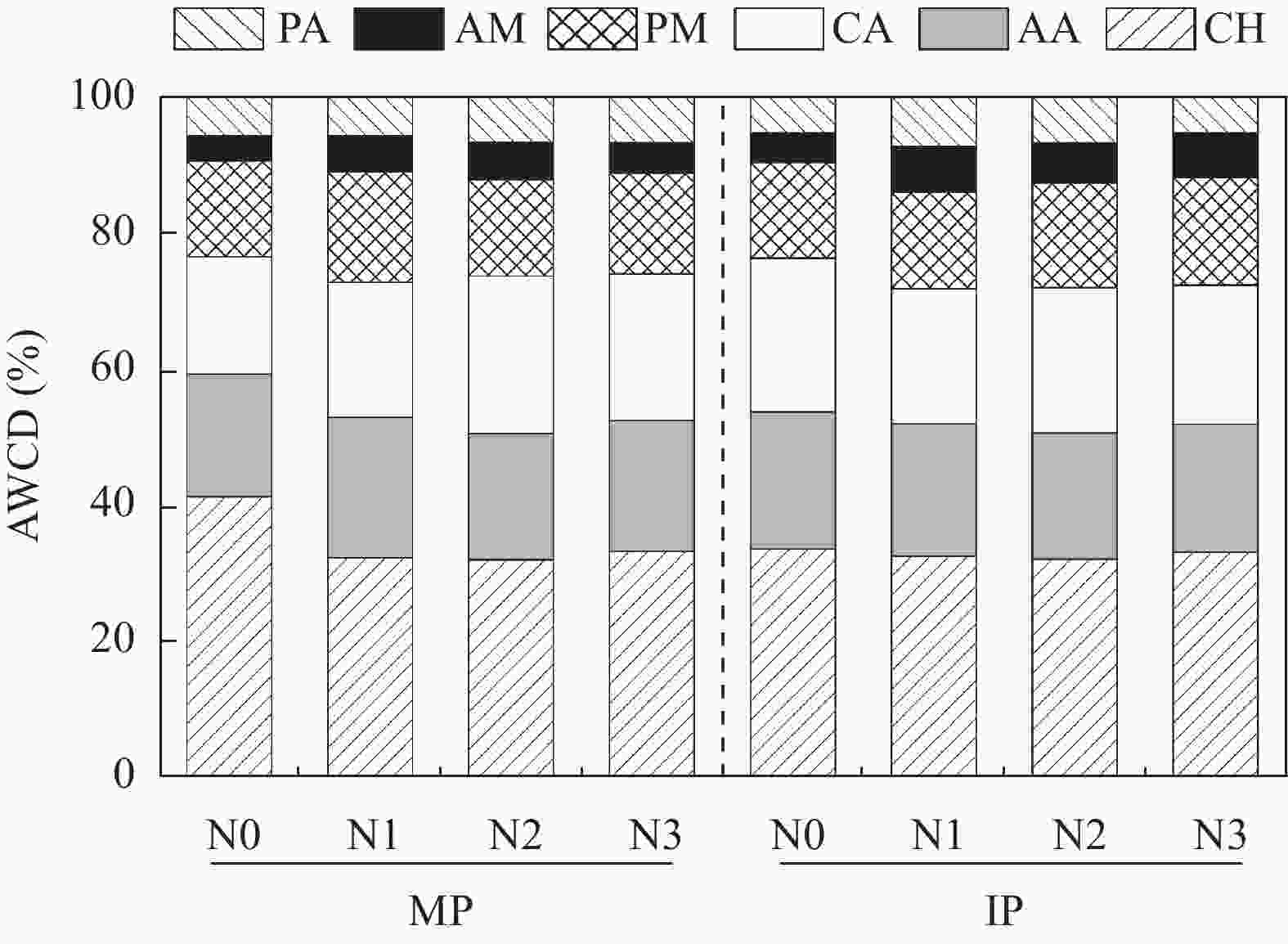
图 6 不同氮水平下单作(MP)和间作(IP)马铃薯土壤中6类碳源利用的相对比例
CH: 碳水化合物; AA: 氨基酸; CA: 羧酸; PM: 聚合物; AM: 胺类; PA: 酚类化合物。N0、N1、N2和N3分别表示马铃薯种植土壤施氮量为0 kg∙hm−2、62.5 kg∙hm−2、125 kg∙hm−2和187.5 kg∙hm−2. CH: carbohydrates; AA: amino acids; CA: carboxylic acids; PM: polymers; AM: amines; PA: phenolic compounds. N application rates of N0, N1, N2, and N3 are 0, 62.5, 125 and 187.5 kg∙hm−2 in potato planted soil, respectively.
Figure 6. Relative proportions of six carbon sources utilization in mono- (MP) and inter-cropped (IP) potato soils under different nitrogen application levels
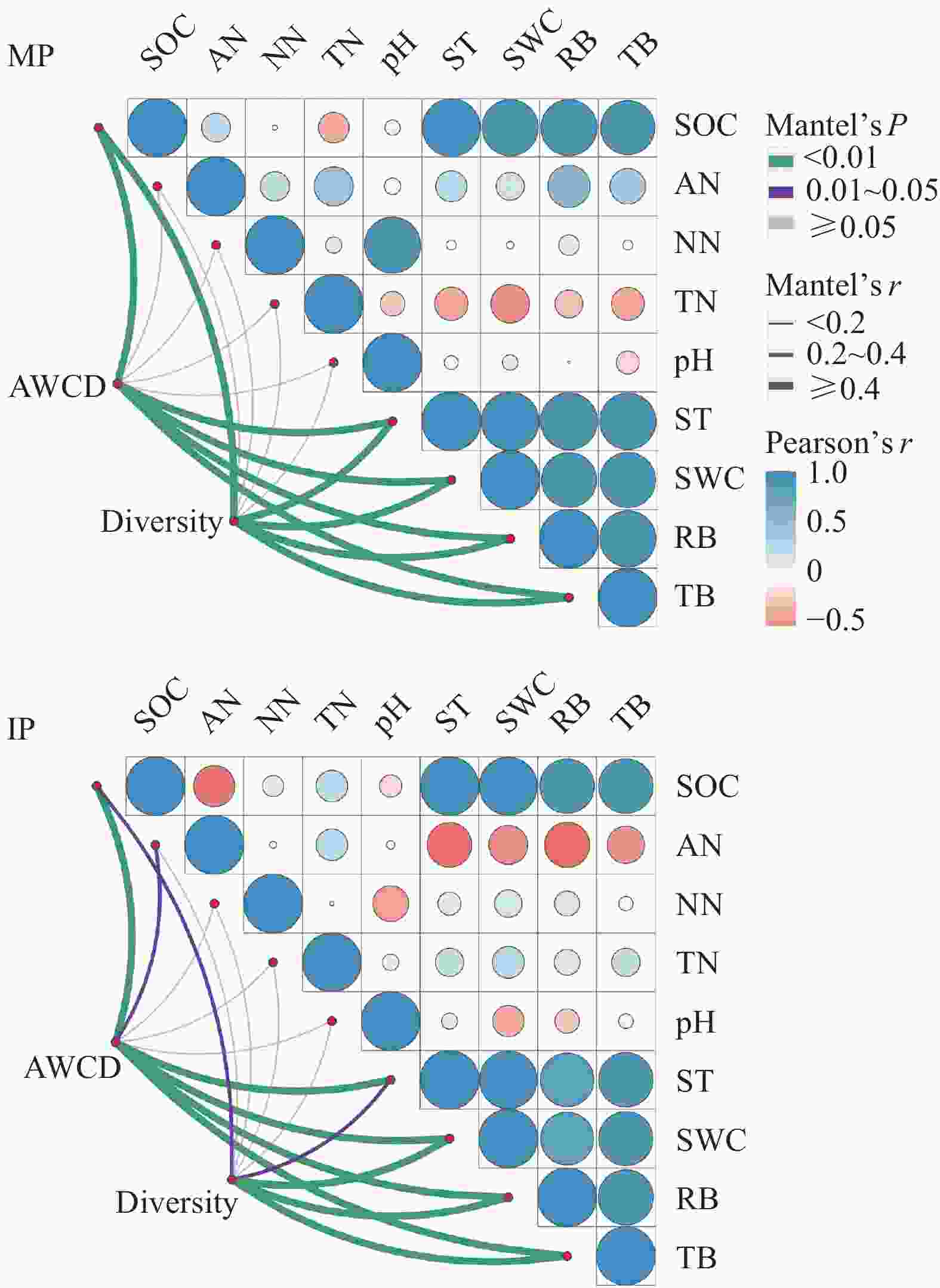
图 7 单作(MP)和间作(IP)马铃薯土壤微生物的AWCD值和功能多样性性指数(Simpson和Shannon指数)与土壤环境因子和马铃薯生物量的关系
连线的宽度对应Mantel检验的r值, 连线的颜色表示统计学上的显著性; 环境因子之间的皮尔逊相关系数用不同颜色梯度表示。SOC: 土壤有机碳; AN: 铵态氮; NN: 硝态氮; TN: 全氮; ST: 土壤温度; SWC: 土壤含水量; RB: 根系生物量; TB: 总生物量。
Figure 7. Relationships between the microbial AWCD value and functional diversity indexes (Simpson and Shannon indexes) with soil environmental factors and potato biomass in mono- (MP) and inter-cropped (IP) potato soils
Edge width corresponds to the Mantel’s r value, and the edge colour denotes the statistical significance. Pearson correlations of environmental factors are shown with a colour gradient denoting Pearson’s correlation coefficients. SOC: soil organic carbon; AN: ammonium nitrogen; NN: nitrate nitrogen; TN: total nitrogen; ST: soil temperature; SWC: soil water content; RB: root biomass; TB: total biomass.
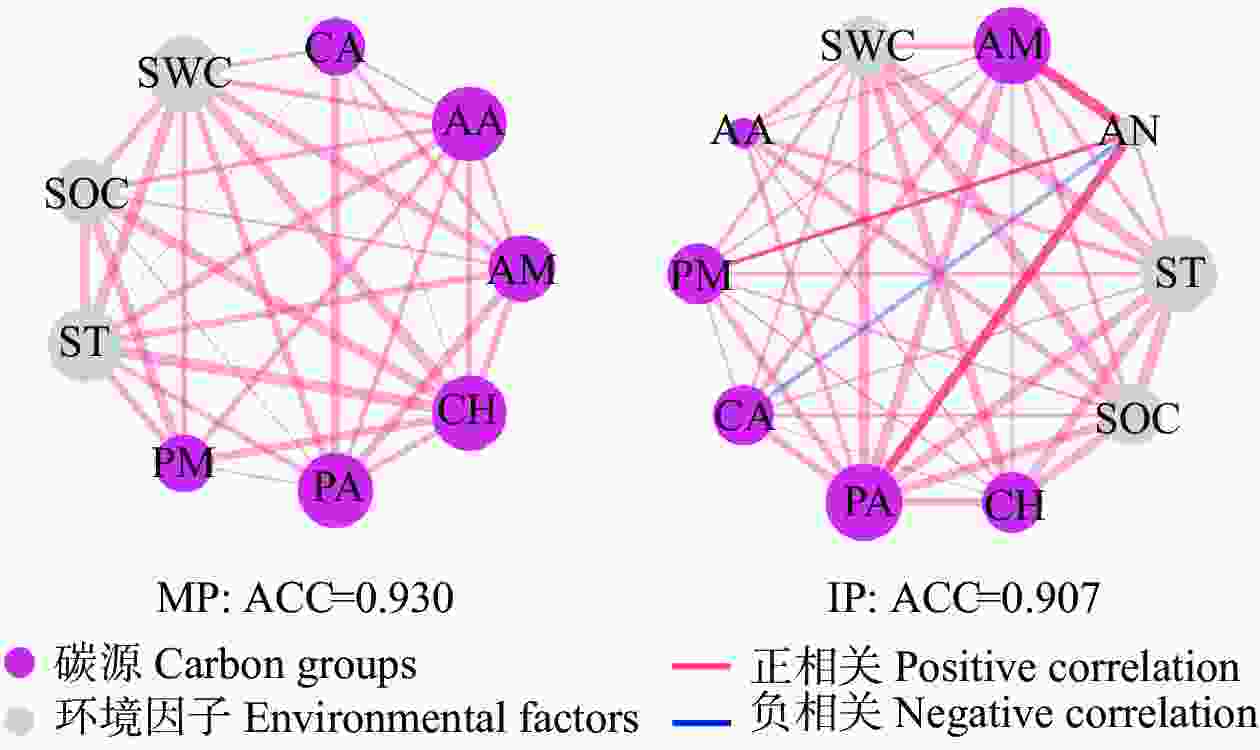
图 8 马铃薯单作(MP)和间作(IP)种植土壤中主要环境因子与不同碳源的相关性网络
连线表示两个变量之间的显著相关性(P<0.05)。每个节点的大小与连接的数量呈正相关, 并且两个节点之间的每个连接的宽度与Pearson相关系数呈正相关。ACC: 平均聚类系数; CH: 碳水化合物; AA: 氨基酸; CA: 羧酸; PM: 聚合物; AM: 胺类; PA: 酚类化合物; SOC: 土壤有机碳; ST: 土壤温度; SWC: 土壤含水量; AN: 土壤铵态氮。
Figure 8. Correlation networks between major environmental factors and varied carbon groups in mono- (MP) and inter-cropped (IP) potato soils
A connection stands for a significant (P<0.05) correlation between two variables. The size of each node is proportional to the number of connections, and the thickness of each connection between two nodes is proportional to the Pearson correlation coefficients. ACC: average clustering coefficients. CH: carbohydrates; AA: amino acids; CA: carboxylic acids; PM: polymers; AM: amines; PA: phenolic compounds; SOC: soil organic carbon; ST: soil temperature; SWC: soil water content; AN: ammonium nitrogen.
表 1 不同氮水平下马铃薯单作(MP)和间作(IP)土壤的理化性状
Table 1. Soil physicochemical properties of mono- (MP) and inter-cropped (IP) potato under different nitrogen application levels
种植模式
Cropping pattern氮水平
Nitrogen level土壤有机碳
Soil organic carbon
(g∙kg−1)硝态氮
Nitrate nitrogen
(mg∙kg−1)铵态氮
Ammonium nitrogen
(mg∙kg−1)pH 土壤温度
Soil temperature
(℃)土壤含水量
Soil water content
(%)MP N0 9.87±0.33c 9.05±0.82c 1.25±0.50c 6.48±0.17a 20.95±1.25c 22.05±2.02b N1 12.25±0.54a 13.15±0.40a 4.25±0.32a 6.96±0.15a 24.35±1.03a 25.03±1.07a N2 11.02±0.69b 12.02±0.92b 3.85±0.13b 6.66±0.20a 22.35±1.05b 22.32±1.32b N3 10.51±0.76b 11.34±0.65b 2.87±0.16b 6.71±0.08a 22.08±0.93b 22.07±1.06b IP N0 10.93±0.36c* 9.98±0.55b 1.45±0.10c 6.47±0.13a 21.07±0.87b 23.65±0.26b* N1 13.10±0.72a 12.01±0.58a 4.58±1.03a 6.60±0.20a 23.05±0.62a 25.01±1.03a N2 12.05±0.52b 11.25±0.60a 4.05±0.95a 6.39±0.16a 21.85±1.23ab 24.06±0.96ab N3 11.02±1.10c 10.56±1.03ab 3.57±0.32ab 6.55±0.23a 21.65±2.01ab 23.97±0.53b 不同小写字母表示同一种植模式下的不同氮水平存在显著差异(P<0.05); *表示相同氮水平下不同种植模式存在显著差异(P<0.05)。N0、N1、N2和N3分别表示马铃薯种植土壤的施氮量为0 kg∙hm−2、62.5 kg∙hm−2、125 kg∙hm−2和187.5 kg∙hm−2. Different lowercase letters mean significant differences among different nitrogen levels at the same cropping pattern at P<0.05. * means significant difference between two cropping patterns under the same nitrogen level at P<0.05. N application rates of N0, N1, N2, and N3 are 0, 62.5, 125 and 187.5 kg∙hm−2 in potato planted soil, respectively. 表 2 不同氮水平下马铃薯单作和间作种植土壤微生物AWCD值和多样性指数的多因素方差分析
Table 2. Multivariate analysis on AWCD value and diversity indexes of soil microorganism in potato mono- and inter-cropping system under different nitrogen application levels
影响因子 Impact factor AWCD Simpson Shannon 氮水平 Nitrogen level (N) *** *** ** 种植模式 Cropping pattern (C) *** ** * N × C NS NS NS NS: 无显著性差异; ***: P<0.001; **: P<0.01; *: P<0.05; NS: no significant. -
[1] LI C, LIU X, MENG M J, et al. The use of Biolog Eco microplates to compare the effects of sulfuric and nitric acid rain on the metabolic functions of soil microbial communities in a subtropical plantation within the Yangtze River Delta region[J]. CATENA, 2021, 198: 105039 doi: 10.1016/j.catena.2020.105039 [2] WANG X Y, BIAN Q, JIANG Y J, et al. Organic amendments drive shifts in microbial community structure and keystone taxa which increase C mineralization across aggregate size classes[J]. Soil Biology and Biochemistry, 2021, 153: 108062 doi: 10.1016/j.soilbio.2020.108062 [3] LIN Y X, YE G P, KUZYAKOV Y, et al. Long-term manure application increases soil organic matter and aggregation, and alters microbial community structure and keystone taxa[J]. Soil Biology and Biochemistry, 2019, 134: 187−196 doi: 10.1016/j.soilbio.2019.03.030 [4] BAO Y Y, FENG Y Z, STEGEN J C, et al. Straw chemistry links the assembly of bacterial communities to decomposition in paddy soils[J]. Soil Biology and Biochemistry, 2020, 148: 107866 doi: 10.1016/j.soilbio.2020.107866 [5] TRIVEDI C, DELGADO-BAQUERIZO M, HAMONTS K, et al. Losses in microbial functional diversity reduce the rate of key soil processes[J]. Soil Biology and Biochemistry, 2019, 135: 267−274 doi: 10.1016/j.soilbio.2019.05.008 [6] MAN J, TANG B, XING W, et al. Root litter diversity and functional identity regulate soil carbon and nitrogen cycling in a typical steppe[J]. Soil Biology and Biochemistry, 2020, 141: 107688 doi: 10.1016/j.soilbio.2019.107688 [7] GONG X W, LIU C J, LI J, et al. Responses of rhizosphere soil properties, enzyme activities and microbial diversity to intercropping patterns on the Loess Plateau of China[J]. Soil and Tillage Research, 2019, 195: 104355 doi: 10.1016/j.still.2019.104355 [8] HE X H, ZHU S S, WANG H N, et al. Crop diversity for ecological disease control in potato and maize[J]. Journal of Resources and Ecology, 2010, 1(1): 45−50 [9] YIN W, CHAI Q, GUO Y, et al. Reducing carbon emissions and enhancing crop productivity through strip intercropping with improved agricultural practices in an arid area[J]. Journal of Cleaner Production, 2017, 166: 197−208 doi: 10.1016/j.jclepro.2017.07.211 [10] CONG W F, HOFFLAND E, LI L, et al. Intercropping enhances soil carbon and nitrogen[J]. Global Change Biology, 2015, 21(4): 1715−1726 doi: 10.1111/gcb.12738 [11] WANG J X, LU X N, ZHANG J E, et al. Intercropping perennial aquatic plants with rice improved paddy field soil microbial biomass, biomass carbon and biomass nitrogen to facilitate soil sustainability[J]. Soil and Tillage Research, 2021, 208: 104908 doi: 10.1016/j.still.2020.104908 [12] ZHAO M, JONES C M, MEIJER J, et al. Intercropping affects genetic potential for inorganic nitrogen cycling by root-associated microorganisms in Medicago sativa and Dactylis glomerata[J]. Applied Soil Ecology, 2017, 119: 260−266 doi: 10.1016/j.apsoil.2017.06.040 [13] NYAWADE S O, KARANJA N N, GACHENE C K K, et al. Short-term dynamics of soil organic matter fractions and microbial activity in smallholder potato-legume intercropping systems[J]. Applied Soil Ecology, 2019, 142: 123−135 doi: 10.1016/j.apsoil.2019.04.015 [14] WANG D, YI W B, ZHOU Y L, et al. Intercropping and N application enhance soil dissolved organic carbon concentration with complicated chemical composition[J]. Soil and Tillage Research, 2021, 210: 104979 doi: 10.1016/j.still.2021.104979 [15] WANG Y, ZHANG C, ZHANG G N, et al. Carbon input manipulations affecting microbial carbon metabolism in temperate forest soils — A comparative study between broadleaf and coniferous plantations[J]. Geoderma, 2019, 355: 113914 doi: 10.1016/j.geoderma.2019.113914 [16] CROW S E, LAJTHA K, BOWDEN R D, et al. Increased coniferous needle inputs accelerate decomposition of soil carbon in an old-growth forest[J]. Forest Ecology and Management, 2009, 258(10): 2224−2232 doi: 10.1016/j.foreco.2009.01.014 [17] XU Y H, FAN J L, DING W X, et al. Characterization of organic carbon in decomposing litter exposed to nitrogen and sulfur additions: Links to microbial community composition and activity[J]. Geoderma, 2017, 286: 116−124 doi: 10.1016/j.geoderma.2016.10.032 [18] 李秋梅, 黎胜杰, 王欣丽, 等. 改变碳输入对沂蒙山区典型次生林土壤微生物碳源代谢功能的影响[J]. 生态学报, 2021, 41(10): 4110−4119LI Q M, LI S J, WANG X L, et al. Influences of changing carbon inputs on soil microbial carbon metabolism in natural secondary forests in Yimeng Mountainous area[J]. Acta Ecologica Sinica, 2021, 41(10): 4110−4119 [19] HANDA I T, AERTS R, BERENDSE F, et al. Consequences of biodiversity loss for litter decomposition across biomes[J]. Nature, 2014, 509(7499): 218−221 doi: 10.1038/nature13247 [20] SANTONJA M, RANCON A, FROMIN N, et al. Plant litter diversity increases microbial abundance, fungal diversity, and carbon and nitrogen cycling in a Mediterranean shrubland[J]. Soil Biology and Biochemistry, 2017, 111: 124−134 doi: 10.1016/j.soilbio.2017.04.006 [21] PORRE R J, VAN DER WERF W, DE DEYN G B, et al. Is litter decomposition enhanced in species mixtures? A meta-analysis[J]. Soil Biology and Biochemistry, 2020, 145: 107791 doi: 10.1016/j.soilbio.2020.107791 [22] LUO R Y, KUZYAKOV Y, LIU D Y, et al. Nutrient addition reduces carbon sequestration in a Tibetan grassland soil: Disentangling microbial and physical controls[J]. Soil Biology and Biochemistry, 2020, 144: 107764 doi: 10.1016/j.soilbio.2020.107764 [23] 董艳, 杨智仙, 董坤, 等. 施氮水平对蚕豆枯萎病和根际微生物代谢功能多样性的影响[J]. 应用生态学报, 2013, 24(4): 1101−1108DONG Y, YANG Z X, DONG K, et al. Effects of nitrogen application rate on faba bean Fusarium wilt and rhizospheric microbial metabolic functional diversity[J]. Chinese Journal of Applied Ecology, 2013, 24(4): 1101−1108 [24] 赵薇, 伊文博, 王顶, 等. 间作对马铃薯种植土壤硝化作用和硝态氮供应的影响[J]. 应用生态学报, 2020, 31(12): 4171−4179ZHAO W, YI W B, WANG D, et al. Effects of intercropping on soil nitrification and nitrogen supply in potato field[J]. Chinese Journal of Applied Ecology, 2020, 31(12): 4171−4179 [25] WU K X, FULLEN M A, AN T X, et al. Above- and below-ground interspecific interaction in intercropped maize and potato: a field study using the ‘target’ technique[J]. Field Crops Research, 2012, 139: 63−70 doi: 10.1016/j.fcr.2012.10.002 [26] WANG D, ZHAO P, XIANG R, et al. Nitrogen fertilization overweighs intercropping in promotion of dissolved organic carbon concentration and complexity in potato-cropped soil[J]. Plant and Soil, 2021, 462(1/2): 273−284 [27] 鲍士旦. 土壤农化分析[M]. 3版. 北京: 中国农业出版社, 2000: 42–108BAO S D. Soil and Agricultural Chemistry Analysis[M]. Beijing: Chinese Agriculture Press, 2000: 42–108 [28] MAO Q G, LU X K, MO H, et al. Effects of simulated N deposition on foliar nutrient status, N metabolism and photosynthetic capacity of three dominant understory plant species in a mature tropical forest[J]. Science of the Total Environment, 2018, 610/611: 555−562 doi: 10.1016/j.scitotenv.2017.08.087 [29] LI W B, JIN C J, GUAN D X, et al. The effects of simulated nitrogen deposition on plant root traits: a meta-analysis[J]. Soil Biology and Biochemistry, 2015, 82: 112−118 doi: 10.1016/j.soilbio.2015.01.001 [30] ZHANG K P, DUAN M C, XU Q W, et al. Soil microbial functional diversity and root growth responses to soil amendments contribute to CO2 emission in rainfed cropland[J]. CATENA, 2020, 195: 104747 doi: 10.1016/j.catena.2020.104747 [31] ZHOU S X, BUTENSCHOEN O, BARANTAL S, et al. Decomposition of leaf litter mixtures across biomes: The role of litter identity, diversity and soil fauna[J]. Journal of Ecology, 2020, 108(6): 2283−2297 doi: 10.1111/1365-2745.13452 -





 下载:
下载: