Effect of long-term fertilization on the stabilization of soil organic carbon by iron oxides in red soil
-
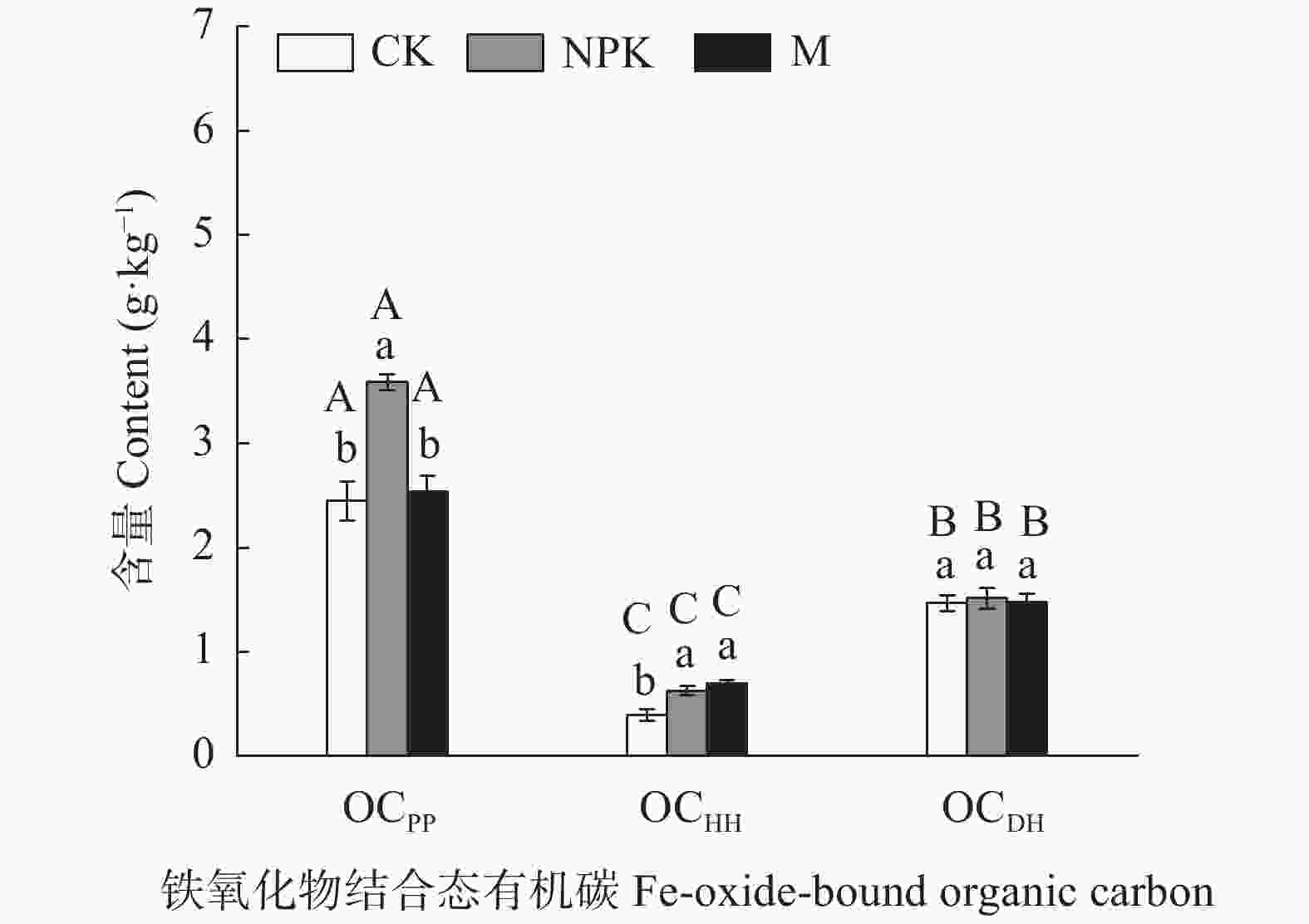
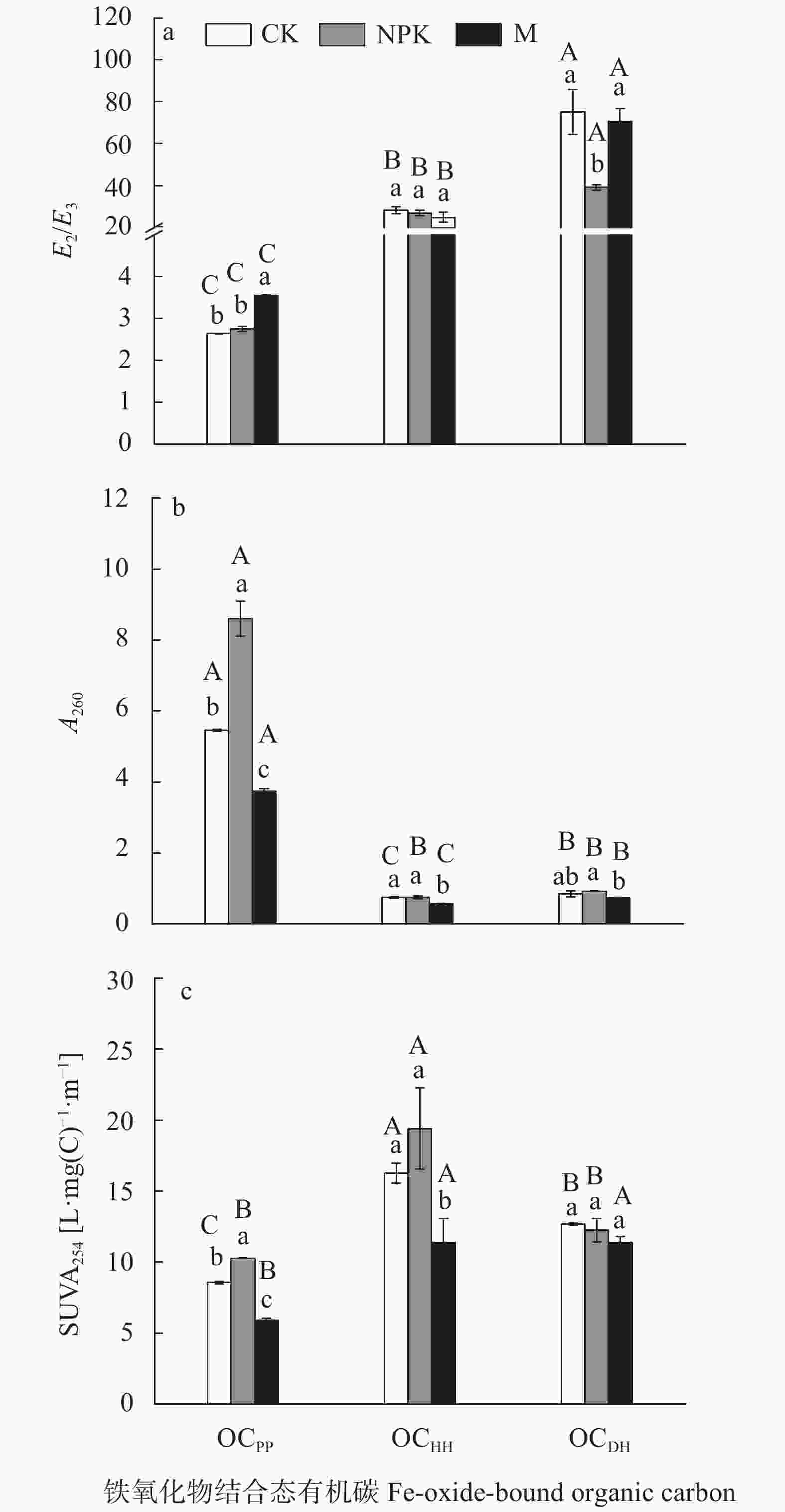
摘要: 以湖南衡阳红壤实验站25年长期定位施用化肥和有机肥的红壤为研究对象, 通过焦磷酸钠、盐酸羟胺和连二亚硫酸钠-盐酸溶液分别提取了土壤中络合铁、非晶形铁氧化物和晶形铁氧化物及其结合态有机碳, 借助总有机碳分析和紫外-可见光谱等技术探讨了红壤中不同类型铁氧化物对土壤有机碳固定的贡献及其对长期施肥的响应。结果表明, 红壤中不同类型铁氧化物结合态有机碳的含量为络合铁结合态有机碳(2.45~3.59 g∙kg–1, OCPP)>晶形铁氧化物结合态有机碳(1.46~1.51 g∙kg–1, OCDH)>非晶形铁氧化物结合态有机碳(0.39~0.70 g∙kg–1, OCHH), 其中OCPP主要是络合铁与芳香性弱、疏水性强的大分子有机物通过螯合或者共沉淀作用形成, 而OCHH和OCDH则主要是吸附在(羟基)铁氧化物上的芳香类化合物, OCHH比OCDH具有更大的分子量和更强的芳香性。长期施用化肥(NPK)显著增加(P<0.05)了红壤中OCHH和OCPP的含量, 长期施用有机肥(M)仅促进(P<0.05)非晶形铁氧化物与有机碳结合。NPK处理显著增加(P<0.05)红壤中OCDH的平均分子量以及OCPP的疏水性和芳香性, 而M处理则降低(P<0.05)了OCPP的平均分子量、OCPP和OCHH的疏水性和芳香性。综上所述, NPK和M处理均能提高红壤中铁氧化物的固碳能力, 但对不同类型的铁氧化物影响不一, 此外长期施肥还会改变铁氧化物结合态有机碳的组成。Abstract: The preservation and decomposition of soil organic carbon (SOC) has been the subject of scientific inquiry for decades, owing to its critical role in regulating atmospheric CO2 concentrations. Iron (Fe) oxides are widely recognized as a rusty sink for carbon (C) because of their large surface area and high adsorption affinity. Fe oxides such as amorphous Fe hydroxides, crystalline Fe hydroxides, and organo-Fe complexes coexist in soils and can be converted to one another. An in-depth understanding of the stabilization of SOC by different types of Fe oxides will strengthen our understanding of soil C cycling. Based on a long-term (25 years) fertilization field experiment in Hengyang, Hunan Province, China, investigations were performed to clarify the stabilization of SOC using different Fe oxides, and its responses to long-term fertilization were discussed. A selective extraction was conducted sequentially to determine the distribution of organic carbon (OC) among different Fe oxides: Na-pyrophosphate (organo-Fe complexes) followed by HCl-hydroxylamine (amorphous Fe hydroxides) and dithionite-HCl (crystalline Fe hydroxides). Ultraviolet and visible light adsorption measurements were used to analyze the composition of Fe oxide-bound OC. The OC contents differed among different Fe oxides in the red soil in the following order: organo-Fe complex-bound OC (2.45–3.59 g∙kg–1, OCPP) > crystalline Fe hydroxide-bound OC (1.46–1.51 g∙kg–1, OCDH) > amorphous Fe hydroxide-bound OC (0.39–0.70 g∙kg–1, OCHH). OCPP was formed by the coprecipitation/chelation of organo-Fe complexes with low aromaticity, high molecular weight, and high hydrophobicity compounds. OCHH and OCDH were primarily formed by Fe hydroxide-adsorbed aromatic compounds. OCHH had greater average molecular weights and higher aromaticity than OCDH. Long-term application of chemical fertilizers (NPK) facilitated (P<0.05) the binding of OC with organo-Fe complexes and amorphous Fe hydroxides. However, organic fertilizer (M) addition solely increased (P<0.05) the association of OC with amorphous Fe hydroxides. In addition, NPK treatments increased (P<0.05) the average molecular weights of OCDH and the hydrophobicity and aromaticity of OCPP. However, M treatments decreased (P<0.05) the average molecular weights of OCPP and the hydrophobicity and aromaticity of OCPP and OCHH. These findings suggest that long-term fertilization may increase the stabilization of SOC by Fe oxides in red soil; however, the response of SOC stabilization by Fe oxides with varying crystallinity to long-term fertilization is different. In addition, long-term fertilizer addition may change the composition of Fe oxide-bound OC.
-
Key words:
- Soil carbon storage /
- Red soil /
- Soil organic carbon /
- Long-term fertilization /
- Iron oxides /
- Mineral-organic complex
-
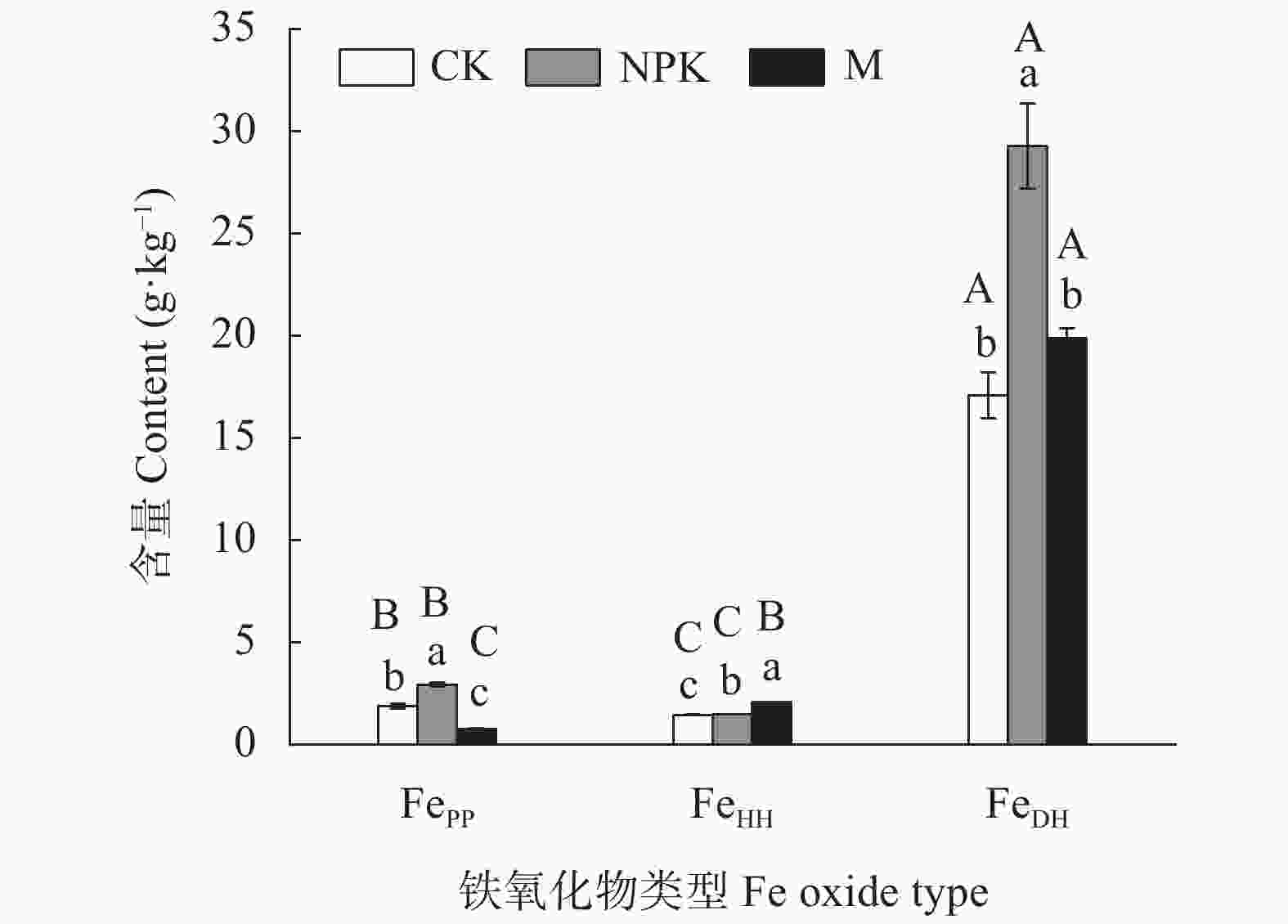
图 1 长期不同施肥处理对土壤中铁氧化物含量的影响
CK、NPK、M分别代表对照、长期施用化肥和长期施用有机肥处理, FePP、FeHH、FeDH分别表示络合铁、非晶形铁氧化物和晶形铁氧化物。不同小写字母和大写字母分别表示不同施肥处理和不同铁氧化物类型间差异显著(P<0.05), 误差线表示标准差(n=3)。
Figure 1. Contents of iron oxides in red soil under different long-term fertilization treatments
CK, NPK and M represent no fertilization, chemical fertilizer application, and manure application treatments, respectively. FePP, FeHH, and FeDH represent organo-Fe complexes, amorphous Fe hydroxides, and crystalline Fe hydroxides, respectively. Different lowercase and capital letters on the graph column indicate significant differences among different treatments for the same iron oxide and among different iron oxides for the same treatment, respectively, at P<0.05 according to Tukey’s multiple range test. Error bar represents the standard deviation of n=3.
图 2 长期不同施肥处理对红壤中铁氧化物结合态有机碳含量的影响
CK、NPK、M分别代表对照、长期施用化肥和长期施用有机肥处理, OCPP、OCHH、OCDH分别表示络合铁结合态有机碳、非晶形铁氧化物结合态有机碳和晶形铁氧化物结合态有机碳。不同小写字母和大写字母分别表示不同施肥处理和不同铁氧化物结合态有机碳类型间差异显著(P<0.05), 误差线表示标准差(n=3)。
Figure 2. Contents of Fe-oxide-bound organic carbon in red soil under different long-term fertilization treatments
CK, NPK, and M represent no fertilization, chemical fertilizer application, and manure application treatments, respectively. OCPP, OCHH, and OCDH represent organo-Fe complexes bound organic carbon, amorphous Fe hydroxides bound organic carbon, and crystalline Fe hydroxides bound organic carbon, respectively. Different lowercase and capital letters on the graph column indicate significant differences among different treatments for the same Fe-oxide-bound organic carbon and among different Fe-oxide-bound organic carbon for the same treatment, respectively, at P<0.05 according to Tukey’s multiple range test. Error bar represents the standard deviation of n=3.
表 1 长期不同施肥处理的红壤pH和总有机碳含量
Table 1. pH and organic carbon content in red soil under different long-term fertilization treatments
处理
TreatmentpH 有机碳含量
Organic carbon content (g∙kg−1)CK 4.85±0.02b 7.0±0.1c NPK 3.77±0.00c 9.4±0.2b M 5.96±0.03a 16.5±0.2a CK、NPK、M分别代表对照、长期施用化肥和长期施用有机肥处理, 同列不同字母表示不同施肥处理间差异显著(P<0.05)。CK, NPK, and M represent no fertilization, chemical fertilizer application, and manure application treatments, respectively. Values in the same column followed by different letters are significantly different (P<0.05). 表 2 长期不同施肥处理下红壤不同铁氧化物结合态有机碳的碳/铁摩尔比
Table 2. C/Fe molar ratios of different Fe-oxide-bound organic carbon under different long-term fertilization treatments
处理 Treatment OCPP OCHH OCDH CK 6.06±0.50bA 1.27±0.11cB 0.40±0.02aC NPK 5.75±0.23 bA 1.98±0.08aB 0.24±0.02cC M 15.95±1.26 aA 1.58±0.05bB 0.35±0.01bC CK、NPK、M分别代表对照、长期施用化肥和长期施用有机肥处理, OCPP、OCHH、OCDH分别表示络合铁结合态有机碳、非晶形铁氧化物结合态有机碳和晶形铁氧化物结合态有机碳。不同小写字母和大写字母分别表示不同施肥处理和不同铁氧化物结合态有机碳类型间差异显著(P<0.05)。CK, NPK, and M represent no fertilization, chemical fertilizer application, and manure application treatments, respectively. OCPP, OCHH, and OCDH represent organo-Fe complexes bound organic carbon, amorphous Fe hydroxides bound organic carbon, and crystalline Fe hydroxides bound organic carbon, respectively. Different lowercase and capital letters in the table indicate significant differences among different treatments for the same Fe-oxide-bound organic carbon and among different Fe-oxide-bound organic carbon for the same treatment, respectively, at P<0.05 according to Tukey’s multiple range test. -
[1] KLEBER M, EUSTERHUES K, KEILUWEIT M, et al. Mineral-organic associations: formation, properties, and relevance in soil environments[J]. Advances in Agronomy, 2015, 130: 1−140 [2] YU G H, XIAO J, HU S J, et al. Mineral availability as a key regulator of soil carbon storage[J]. Environmental Science & Technology, 2017, 51(9): 4960−4969 [3] HEMINGWAY J D, ROTHMAN D H, GRANT K E, et al. Mineral protection regulates long-term global preservation of natural organic carbon[J]. Nature, 2019, 570(7760): 228−231 doi: 10.1038/s41586-019-1280-6 [4] WANG Y Y, WANG H, HE J S, et al. Iron-mediated soil carbon response to water-table decline in an alpine wetland[J]. Nature Communications, 2017, 8: 15972 doi: 10.1038/ncomms15972 [5] KRAMER M G, CHADWICK O A. Climate-driven thresholds in reactive mineral retention of soil carbon at the global scale[J]. Nature Climate Change, 2018, 8(12): 1104−1108 doi: 10.1038/s41558-018-0341-4 [6] 王璐莹, 秦雷, 吕宪国, 等. 铁促进土壤有机碳累积作用研究进展[J]. 土壤学报, 2018, 55(5): 1041−1050 doi: 10.11766/trxb201802260035WANG L Y, QIN L, LYU X G, et al. Progress in researches on effect of iron promoting accumulation of soil organic carbon[J]. Acta Pedologica Sinica, 2018, 55(5): 1041−1050 doi: 10.11766/trxb201802260035 [7] JEEWANI P H, GUNINA A, LIANG T, et al. Rusty sink of rhizodeposits and associated keystone microbiomes[J]. Soil Biology & Biochemistry, 2020, 147: 107840 [8] JEEWANI P H, ZWIETEN L V, ZHU Z K, et al. Abiotic and biotic regulation on carbon mineralization and stabilization in paddy soils along iron oxide gradients[J]. Soil Biology & Biochemistry, 2021, 160: 108312 [9] WAN D, YE T H, LU Y, et al. Iron oxides selectively stabilize plant-derived polysaccharides and aliphatic compounds in agricultural soils[J]. European Journal of Soil Science, 2019, 70(6): 1153−1163 [10] 万丹. 铁氧化物和钙离子对土壤有机碳的固定及有机质对Pb形态转化的影响[D]. 武汉: 华中农业大学, 2019WAN D. Stabilization of soil organic carbon by iron oxides and calcium ions and the effect of organic matter on the fraction of Pb[D]. Wuhan: Huazhong Agricultural University, 2019 [11] KAISER K, GUGGENBERGER G. Mineral surfaces and soil organic matter[J]. European Journal of Soil Science, 2003, 54(2): 219−236 doi: 10.1046/j.1365-2389.2003.00544.x [12] COWARD E K, THOMPSON A T, PLANTE A F. Iron-mediated mineralogical control of organic matter accumulation in tropical soils[J]. Geoderma, 2017, 306: 206−216 doi: 10.1016/j.geoderma.2017.07.026 [13] HECKMAN K, LAWRENCE C R, HARDEN J W. A sequential selective dissolution method to quantify storage and stability of organic carbon associated with Al and Fe hydroxide phases[J]. Geoderma, 2018, 312: 24−35 doi: 10.1016/j.geoderma.2017.09.043 [14] HALL S J, BERHE A A, THOMPSON A. Order from disorder: do soil organic matter composition and turnover co-vary with iron phase crystallinity?[J]. Biogeochemistry, 2018, 140(1): 93−110 doi: 10.1007/s10533-018-0476-4 [15] ZHANG W J, WANG X J, XU M G, et al. Soil organic carbon dynamics under long-term fertilizations in arable land of northern China[J]. Biogeosciences, 2010, 7(2): 409−425 doi: 10.5194/bg-7-409-2010 [16] HUANG C C, LIU S, LI R Z, et al. Spectroscopic evidence of the improvement of reactive iron mineral content in red soil by long-term application of swine manure[J]. PLoS One, 2016, 11(1): e0146364 doi: 10.1371/journal.pone.0146364 [17] WEN Y L, XIAO J, LIU F F, et al. Contrasting effects of inorganic and organic fertilisation regimes on shifts in Fe redox bacterial communities in red soils[J]. Soil Biology and Biochemistry, 2018, 117: 56−67 doi: 10.1016/j.soilbio.2017.11.003 [18] HUANG W J, HALL S J. Elevated moisture stimulates carbon loss from mineral soils by releasing protected organic matter[J]. Nature Communications, 2017, 8: 1774 doi: 10.1038/s41467-017-01998-z [19] CHEN C M, HALL S J, COWARD E, et al. Iron-mediated organic matter decomposition in humid soils can counteract protection[J]. Nature Communications, 2020, 11: 2255 doi: 10.1038/s41467-020-16071-5 [20] YU G H, KUZYAKOV Y. Fenton chemistry and reactive oxygen species in soil: abiotic mechanisms of biotic processes, controls and consequences for carbon and nutrient cycling[J]. Earth-Science Reviews, 2021, 214: 103525 [21] WAN D, MA M K, PENG N, et al. Effects of long-term fertilization on calcium-associated soil organic carbon: implications for C sequestration in agricultural soils[J]. Science of the Total Environment, 2021, 772: 145037 doi: 10.1016/j.scitotenv.2021.145037 [22] WAN D, ZHANG N C, CHEN W L, et al. Organic matter facilitates the binding of Pb to iron oxides in a subtropical contaminated soil[J]. Environmental Science and Pollution Research International, 2018, 25(32): 32130−32139 doi: 10.1007/s11356-018-3173-x [23] SCHNECKER J, BORKEN W, SCHINDLBACHER A, et al. Little effects on soil organic carbon chemistry of density fractions after seven years of forest soil warming[J]. Soil Biology & Biochemistry, 2016, 103: 300−307 [24] 史吉平, 张夫道, 林葆. 长期定位施肥对土壤腐殖质理化性质的影响[J]. 中国农业科学, 2002, 35(2): 174−180 doi: 10.3321/j.issn:0578-1752.2002.02.012SHI J P, ZHANG F D, LIN B. Effects of long-term located fertilization on the physico-chemical property of soil humus[J]. Scientia Agricultura Sinica, 2002, 35(2): 174−180 doi: 10.3321/j.issn:0578-1752.2002.02.012 [25] GAO J K, LIANG C L, SHEN G Z, et al. Spectral characteristics of dissolved organic matter in various agricultural soils throughout China[J]. Chemosphere, 2017, 176: 108−116 doi: 10.1016/j.chemosphere.2017.02.104 [26] CHEN C M, DYNES J J, WANG J, et al. Properties of Fe-organic matter associations via coprecipitation versus adsorption[J]. Environmental Science & Technology, 2014, 48(23): 13751−13759 [27] POULIN B A, RYAN J N, AIKEN G R. Effects of iron on optical properties of dissolved organic matter[J]. Environmental Science & Technology, 2014, 48(17): 10098−10106 [28] DILLING J, KAISER K. Estimation of the hydrophobic fraction of dissolved organic matter in water samples using UV photometry[J]. Water Research, 2002, 36(20): 5037−5044 doi: 10.1016/S0043-1354(02)00365-2 [29] WAGAI R, MAYER L M, KITAYAMA K, et al. Association of organic matter with iron and aluminum across a range of soils determined via selective dissolution techniques coupled with dissolved nitrogen analysis[J]. Biogeochemistry, 2013, 112(1): 95−109 [30] PARFITT R L, CHILDS C W. Estimation of forms of Fe and Al — a review, and analysis of contrasting soils by dissolution and Mossbauer methods[J]. Soil Research, 1988, 26(1): 121 doi: 10.1071/SR9880121 [31] KAISER K, ZECH W. Defects in estimation of aluminum in humus complexes of podzolic soils by pyrophosphate extraction[J]. Soil Science, 1996, 161(7): 452−458 doi: 10.1097/00010694-199607000-00005 [32] REGELINK I C, VOEGELIN A, WENG L P, et al. Characterization of colloidal Fe from soils using field-flow fractionation and Fe K-edge X-ray absorption spectroscopy[J]. Environmental Science & Technology, 2014, 48(8): 4307−4316 [33] LAWRENCE C R, HARDEN J W, XU X M, et al. Long-term controls on soil organic carbon with depth and time: a case study from the Cowlitz River chronosequence, WA USA[J]. Geoderma, 2015, 247/248: 73−87 doi: 10.1016/j.geoderma.2015.02.005 [34] COWARD E K, OHNO T, PLANTE A F. Adsorption and molecular fractionation of dissolved organic matter on iron-bearing mineral matrices of varying crystallinity[J]. Environmental Science & Technology, 2018, 52(3): 1036−1044 [35] HUANG X L, FENG C L, ZHAO G L, et al. Carbon sequestration potential promoted by oxalate extractable iron oxides through organic fertilization[J]. Soil Science Society of America Journal, 2017, 81(6): 1359−1370 doi: 10.2136/sssaj2017.02.0068 [36] LV J T, ZHANG S Z, WANG S S, et al. Molecular-scale investigation with ESI-FT-ICR-MS on fractionation of dissolved organic matter induced by adsorption on iron oxyhydroxides[J]. Environmental Science & Technology, 2016, 50(5): 2328−2336 [37] WENG L P, VAN RIEMSDIJK W H, KOOPAL L K, et al. Ligand and charge distribution (LCD) model for the description of fulvic acid adsorption to goethite[J]. Journal of Colloid and Interface Science, 2006, 302(2): 442−457 doi: 10.1016/j.jcis.2006.07.005 -





 下载:
下载: